Abstract
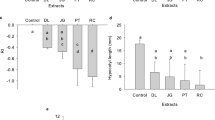
The global rise of herbicide-resistant weeds underscores the urgent need for sustainable weed management strategies. Eupatorium capillifolium (Lam.) Small, a perennial invasive weed native to North America and widespread in the Southeastern United States, presents untapped potential as a bioherbicide. This study evaluated the effects of its aqueous extract on seed germination and early seedling growth of thirteen weed species (nine broadleaf and four grasses) and four major crops (Arachis hypogaea, Zea mays, Glycine max, and Gossypium hirsutum). The extract significantly inhibited seed germination (92.62–100%) of four Amaranthus species (A. palmeri, A. tuberculatus, A. retroflexus, and A. hybridus) with minimal effects on Zea mays and Arachis hypogaea (6.12–6.25%). Other weeds showed a limited response. Inhibition of shoot and root growth confirmed the extract’s allelopathic activity. Principal component analysis indicated inhibition of seed germination as the primary mode of action. The order of pigweeds’ sensitivity to the aqueous extract was A. hybridus > A. retroflexus > A. palmeri > A. tuberculatus. Phytochemical screening identified 36 allelopathic compounds with gallic acid and hydroxy-1,4-benzoquinone as the dominant components. This is the first report demonstrating the bioherbicidal potential of E. capillifolium aqueous extract against Amaranthus spp. under laboratory conditions, highlighting its promise as a sustainable alternative to synthetic herbicides and a candidate for further field-based evaluation in integrated weed management systems.
Similar content being viewed by others
Data availability
All data generated in this experiment are presented including a supplementary file.
References
Weed Science Society of America. Weed impacts on crop yields. https://wssa.net/resources/weed-impacts-on-crop-yields/ (2024).
Llewellyn, R. S., Ronning, D., Ouzman, J., Walker, S., Mayfield, A., & Clarke, M. Impact of weeds on Australian grain production: The cost of weeds to Australian grain growers and the adoption of weed management and tillage practices. Report for GRDC. CSIRO, Australia https://grdc.com.au/__data/assets/pdf_file/0017/622034/impact-of-weeds-australian-grain-production-grdc-20250610.pdf (2016).
Gharde, Y., Singh, P. K., Dubey, R. P. & Gupta, P. K. Assessment of yield and economic losses in agriculture due to weeds in India. Crop Prot. 107, 12–18 (2018).
Heap, I. The international herbicide-resistant weed database. www.weedscience.org (2025).
Moyer, J., Smith, E., Rui, Y. & Hayden, J. Regenerative agriculture and the soil carbon solution. https://rodaleinstitute.org/wp-content/uploads/Rodale-Soil-Carbon-White-Paper_v11-compressed.pdf. (2020)
Peng, G. & Byer, K. N. Interactions of Pyricularia setariae with herbicides for control of green foxtail (Setaria viridis). Weed Technol. 19, 589–598 (2005).
Díaz-Hernández, S., Gallo-Lobet, L., Domínguez-Correa, P. & Rodríguez, A. Effect of repeated cycles of soil solarization and biosolarization on corky root, weeds and fruit yield in screen-house tomatoes under subtropical climate conditions in the Canary Islands. Crop Prot. 94, 20–27 (2017).
Katan, J. & Gamliel, A. Soil Solarization for the Management of Soilborne Pests: The Challenges, Historical Perspective, and Principles (ed. Gamliel, A. & Katan, J.) 45–52 (American Phytopathological Society, 2012).
Aldrich, R. J. & Kremer, R. J. Principles in Weed Management (Iowa State University Press, 1997).
Cordeau, S., Triolet, M., Wayman, S., Steinberg, C. & Guillemin, J. P. Bioherbicides: Dead in the water? A review of the existing products for integrated management. Crop Prot. 87, 44–49 (2016).
VMR. Bioherbicides market by source (microbial bioherbicides, biochemical bioherbicides), mode of action (contact bioherbicides, systemic bioherbicides), application method (foliar spray, soil application), & region for 2024–2031. https://www.verifiedmarketresearch.com/product/bioherbicides-market/ (2024).
Duke, S. O., Pan, Z., Bajsa-Hirshel, J. & Boyette, C. D. The potential future roles oof natural compounds and microbial bioherbicides in weed management in crops. Adv. Weed Sci. 40, e020210054. https://doi.org/10.51694/AdvWeedSci/2022 (2022).
Kremer, R. J. Bioherbicides and Nanotechnology: Current Status and Future Trends (ed. Koul, O.) 353–366 (Academic Press, 2019).
Cimmino, A., Masi, M., Evidente, M., Superchi, S. & Evidente, A. Fungal phytotoxins with potential herbicidal activity: Chemical and biological characterization. Nat. Prod. Rep. 32, 1629–1653 (2015).
Christians, N. E., Liu, D. & Unruh, J. B. The Use of Protein Hydrolysates for Weed Control (ed. Pasupuleti, V. K. & Demain, A. L.) 127–133 (Springer, 2010).
Boydston, R. A., Collins, H. P. & Vaughn, S. F. Response of weeds and ornamental plants to potting soil amended with dried distillers grains. Hort. Science. 43, 191–195 (2008).
Duke, S. O. et al. Chemicals from nature for weed management. Weed Sci. 50, 138–151 (2002).
Shrestha, A. Potential of a black walnut (Juglans nigra) extract product (NatureCur!) as a pre- and post-emergence bioherbicide. J. Sustain. Agric. 33, 810–822 (2009).
Hasan, M. et al. Weed control efficacy and crop-weed selectivity of a new bioherbicide WeedLock. Agronomy 11, 1488. https://doi.org/10.3390/agronomy11081488 (2021).
Ho, T. L. et al. Rice byproducts reduce seed and seedling survival of Echinochloa crusgalli, Leptochloa chinensi, and Fymbristyulis miliacea. Agronomy 11, 776. https://doi.org/10.3390/agr.onomy11040776 (2021).
Dayan, F. E. & Duke, S. O. Natural compounds as next-generation herbicides. Plant Physiol. 166, 1090–1105 (2014).
Kaur, S., Singh, H. P., Mittal, S., Batish, D. R. & Kohli, R. K. Phytotoxic effects of volatile oil from Artemisia scoparia against weeds and its possible use as a bioherbicide. Ind. Crops Prod. 32, 54–61 (2010).
Hazrati, H., Saharkhiz, M. J., Niakousari, M. & Moein, M. Natural herbicide activity of Satureja hortensis L. essential oil nanoemulsion on the seed germination and morphophysiological features of two important weed species. Ecotoxicol. Environ. Saf. 142, 423–430 (2017).
Verdeguer, M., Sanchez-Moreiras, A. M. & Araniti, F. Phytotoxic effects and mechanism of action of essential oils and terpenoids. Plant. 9, 1571. https://doi.org/10.3390/plants9111571 (2020).
Lee, D. L. et al. The discovery and structural requirements of inhibitors of p-hydroxyphenylpyruvate dioxygenase. Weed Sci. 45, 601–609 (1997).
Liu, P. Y. et al. Chemical constituents of plants from the genus Eupatorium (1904–2014). Chem. Biodivers. 12, 1481–1515 (2015).
Kundu, A. et al. Cadinene sesquiterpenes from Eupatorium adenophorum and their antifungal activity. J. Environ. Sci. Health Part B. 48, 516–522 (2013).
Tabanca, N. et al. Eupatorium capillifolium essential oil: Chemical composition, antifungal activity, and insecticidal activity. Nat. Prod. Commun. 5, 1409–1415 (2010).
Ahluwalia, V. et al. Chemical analysis of essential oils of Eupatorium adenophorum and their antimicrobial, antioxidant and phytotoxic properties. J. Pest. Sci. 87, 341–349 (2014).
Sellers, B. A., Ferrell, J. A., MacDonald, G. E. & Kline, W. N. Dogfennel (Eupatorium capillifolium) size at application affects herbicide efficacy. Weed Technol. 23, 247–250 (2009).
USDA NAAS. Census of Agriculture farms, land in farms, value in land and buildings, and farm use: 2002 and 1997 https://agcensus.library.cornell.edu/census_parts/2002-united-states/ (2002).
Crowder, S. H., Cole, A. W. & Watson, V. H. Weed control and forage quality in tebuthiuron treated pastures. Weed Sci. 31, 585–587 (1982).
Rao, K. V. & Alvarez, F. M. Antibiotic principle of Eupatorium capillifolium. J. Nat. Prod. 44, 252–256 (1981).
Okunade, A. & Wiemer, D. F. Ant-repellent sesquiterpene lactones from Eupatorium quadrangularae. Phytochem. 24, 1199–1201 (1985).
Lancelle, H. G., Giordano, O. S., Sosa, M. E. & Tonn, C. E. Chemical composition of four essential oils from Eupatorium spp. biological activities toward Tribolium castaneum (Coleoptera: Tenebrionidae). Rev. Soc. Entomol. Argentina. 68, 329–338 (2009).
Sosa, M. E., Lancelle, H. G., Tonn, C. E., Andres, M. F. & Gonzalez-Coloma, A. Insecticidal and nematicidal essential oils from Argentinean Eupatorium and Baccharis spp. Biochem. Syst. Ecol. 43, 132–138 (2012).
Hollis, C. A., Smith, J. E. & Fisher, R. F. Allelopathic effects of common understory species on germination and growth of southern pines. For. Sci. 28, 509–515 (1982).
Smith, A. E. Potential allelopathic influence of certain pasture weeds. Crop Prot. 9, 410–414 (1990).
WSSA. WSSA survey ranks most common and most troublesome weeds in broadleaf crops, fruits and vegetables, https://wssa.net/2017/05/wssa-survey-ranks-most-common-and-most-troublesome-weeds-in-broadleaf-crops-fruits-and-vegetables/ (2017).
Dai, L. et al. Effects of water extracts of Flaveria bidentis on the seed germination and seedling growth of three plants. Sci. Rep. 12, 1–7. https://doi.org/10.1038/s41598-022-22527-z (2022).
Williamson, G. B. & Richardson, D. Bioassays for allelopathy: Measuring treatment responses with independent controls. J. Chem. Ecol. 14, 181–187 (1988).
Macías, F. A., Mejías, F. J. & Molinillo, J. M. Recent advances in allelopathy for weed control: From knowledge to applications. Pest Manag. Sci. 75, 2413–2436 (2019).
Ghosh, R. K., Price, A. J. & Maity, A. Allelopathic effects of horseweed (Erigeron canadensis) on germination and growth of seven common weeds of the southern United States. Weed Sci. 73, e63. https://doi.org/10.1017/wsc.2025.10034 (2025).
Rice, E. L. Allelopathy—An overview (ed. Cooper-Driver, G. A., Swain, T. & Conn, E. E.) 81–99 (Springer, 1985).
Liu, Y. J., Meng, Z. J., Dang, X. H., Song, W. J. & Zhai, B. Allelopathic effects of Stellera chamaejasme on seed germination and seedling growth of alfalfa and two forage grasses. Acta Pratacult. Sin. 28, 130–138 (2019).
Lopes, R. W., Marques Morais, E., Lacerda, J. J. & Araújo, F. D. Bioherbicidal potential of plant species with allelopathic effects on the weed Bidens bipinnata L. Sci. Rep. 12, 1–12 (2022).
Yu, J. W., Lee, J. H., Song, M. H. & Keum, Y. S. Metabolomic responses of lettuce (Lactuca sativa) to allelopathic benzoquinones from Iris sanguinea seeds. J. Agric. Food Chem. 71, 5143–5153 (2023).
Espinosa-Colín, M. et al. Evaluation of propiophenone, 4-methylacetophenone and 2′,4′-dimethylacetophenone as phytotoxic compounds of labdanum oil from Cistus ladanifer L. Plant. 12, 1187. https://doi.org/10.3390/plants12051187 (2023).
Wu, L., Guo, X. & Harivandi, M. Allelopathic effects of phenolic acids detected in buffalograss (Buchloe dactyloides) clippings on growth of annual bluegrass (Poa annua) and buffalograss seedlings. Environ. Exp. Bot. 39, 159–167 (1998).
Akbar, M. et al. Isolation of herbicidal compounds, quercetin and β-caryophyllene, from Digera muricata. Arab. J. Chem. 16, 104653 (2023).
Khan, A. M. & Khan, M. I. Chemical ecology of capsidiol and its role in plant-plant interactions. Plant Ecol. 219, 1–10 (2018).
Batish, D. R., Singh, H. P., Kaur, S., Kohli, R. K. & Yadav, S. S. Caffeic acid affects early growth, and morphogenetic response of hypocotyl cuttings of mung bean (Phaseolus aureus). J. Plant Physiol. 165, 297–305 (2008).
Nishihara, E., Parvez, M. M., Araya, H., Kawashima, S. & Fujii, Y. L-3-(3,4-Dihydroxyphenyl)alanine (L-DOPA), an allelochemical exuded from velvetbean (Mucuna pruriens) roots. Plant Growth Regul. 45, 113–120 (2005).
Rudrappa, T., Bonsall, J., Gallagher, J. L. & Bais, H. P. Root-secreted allelochemical in the noxious weed Phragmites australis deploys a reactive oxygen species response and microtubule assembly disruption to execute rhizotoxicity. J. Chem. Ecol. 33, 1898–1918 (2007).
Rice, E. L. Allelopathy-an update. Bot. Rev. 45, 15–109 (1979).
Hussain, M. I., El-Sheikh, M. A. & Reigosa, M. J. Allelopathic potential of aqueous extract from Acacia melanoxylon R. Br on Lactuca sativa. Plants 9, 1228. https://doi.org/10.3390/plants9091228 (2020).
Gulzar, A., Siddiqui, M. B. & Bi, S. Phenolic acid allelochemicals induced morphological, ultrastructural, and cytological modification on Cassia sophera L. and Allium cepa L. Protoplasma 253, 1211–1221 (2016).
Blum, U., Shafer, S. R. & Lehman, M. E. Evidence for inhibitory allelopathic interactions involving phenolic acids in field soils: Concepts vs an experimental model. Crit. Rev. Plant Sci. 18, 673–693 (1999).
Shajib, M. T. I., Pedersen, H. A., Mortensen, A. G., Kudsk, P. & Fomsgaard, I. S. Phytotoxic effect, uptake, and transformation of Biochanin A in selected weed species. J. Agric. Food Chem. 60, 10715–10722 (2012).
Upretee, P., Bandara, M. S. & Tanino, K. K. The role of seed characteristics on water uptake preceding germination. Seeds 3, 559–574 (2024).
Korres, N. E. et al. Seedbank persistence of Palmer amaranth (Amaranthus palmeri) and waterhemp (Amaranthus tuberculatus) across diverse geographical regions in the United States. Weed Sci. 66, 446–456 (2018).
Lamont, B. B., Gómez Barreiro, P. & Newton, R. J. Seed-coat thickness explains contrasting germination responses to smoke and heat in Leucadendron. Seed Sci. Res. 32, 70–77 (2022).
Jovanović, A. A. et al. Optimization of the extraction process of polyphenols from Thymus serpyllum L. herb using maceration, heat- and ultrasound-assisted techniques. Sep. Purif. Technol. 179, 369–380 (2017).
Allen, E. & Alvarez, S. International rules for seed testing 2020 (The International Seed Testing Association, Bassersdorf, 2020).
Ritz, C., Baty, F., Streibig, J. C. & Gerhard, D. Dose–response analysis using R. PLoS ONE 10, e0146021. https://doi.org/10.1371/journal.pone.0146021 (2015).
Acknowledgements
The authors thankfully acknowledge the startup funds provided by Auburn University to the corresponding author.
Funding
Startup funds provided by Auburn University to the corresponding author is thankfully acknowledged.
Author information
Authors and Affiliations
Contributions
Rakesh Kumar Ghosh: Writing – original draft, Investigation, Data curation, Formal analysis, Conceptualization; Andrew J. Price: Writing – review & editing; Melissa Boersma: Methodology, Data curation; Aniruddha Maity: Writing – review & editing, Supervision, Resources, Project administration, Funding acquisition, Conceptualization.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ghosh, R.K., Price, A.J., Boersma, M. et al. Unveiling the bioherbicidal potential of Eupatorium capillifolium (Lam.) Small for selective management of agricultural weeds. Sci Rep (2026). https://doi.org/10.1038/s41598-026-37110-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-37110-z