Abstract
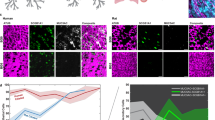
Multiciliated airway epithelial cells possess motile cilia, which are essential for mucociliary clearance, facilitating the removal of particulates from the respiratory system. Previous studies showed that exposure to cigarette toxins causes damage to motile cilia formation, length, and function, and can lead to reduced mucociliary clearance and lung diseases like COPD. Given the limited options for treating smoking-related diseases, it is imperative to define novel therapeutics to address this need. Recently, we discovered that, contrary to its ability to depolymerize microtubules, the small molecule MI-181 can induce ciliogenesis and increase the length of primary cilia in retinal pigment epithelial cells without adverse effects on cell health. Here, we utilized a human airway basal stem cell derived air-liquid interface mucociliary airway epithelium model system, coupled with smoke exposure, to test the effect of MI-181 on motile cilia. We determined that MI-181 promotes the recovery of motile cilia length. Additionally, the effect of MI-181 on the area covered by motile cilia and levels of the FOXJ1 motile cilia transcription factor showed inter-donor heterogeneity. Importantly, transmission electron microscopy analysis of motile cilia axonemes showed that MI-181-treated motile cilia displayed a normal 9 + 2 arrangement of microtubules. Together, these data suggest that MI-181 promotes the recovery of motile cilia length after smoke exposure and that these cilia are structurally intact.
Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. The accompanying Supplemental Material includes Supplemental Table S1.
References
Perotin, J. M. et al. Alteration of primary cilia in COPD. Eur Respir J 52. (2018).
Petit, L. M. G. et al. G., and Airway ciliated cells in adult lung homeostasis and COPD. Eur Respir Rev 32. (2023).
Lee, L. & Ostrowski, L. E. Motile cilia genetics and cell biology: big results from little mice. Cell. Mol. Life Sci. 78, 769–797 (2021).
Bustamante-Marin, X. M. & Ostrowski, L. E. Cilia and mucociliary clearance. Cold Spring Harb Perspect. Biol 9. (2017).
Gohy, S. et al. Altered generation of ciliated cells in chronic obstructive pulmonary disease. Sci. Rep. 9, 17963 (2019).
Leopold, P. L. et al. Smoking is associated with shortened airway cilia. PLoS One. 4, e8157 (2009).
Wynder, E. L., Goodman, D. A. & Hoffmann, D. Ciliatoxic components in cigarette smoke. 3. In vitro comparison of different smoke components, Cancer 18, 1652–1658. (1965).
Park, H. R. et al. Transcriptomic response of primary human airway epithelial cells to flavoring chemicals in electronic cigarettes. Sci. Rep. 9, 1400 (2019).
Ghosh, B. et al. Effect of sub-chronic exposure to cigarette smoke, electronic cigarette and waterpipe on human lung epithelial barrier function. BMC Pulm Med. 20, 216 (2020).
Lechtreck, K. Cargo adapters expand the transport range of intraflagellar transport. J Cell. Sci 135. (2022).
Hessel, J. et al. Intraflagellar transport gene expression associated with short cilia in smoking and COPD. PLoS One. 9, e85453 (2014).
Brekman, A., Walters, M. S., Tilley, A. E. & Crystal, R. G. FOXJ1 prevents cilia growth Inhibition by cigarette smoke in human airway epithelium in vitro. Am. J. Respir Cell. Mol. Biol. 51, 688–700 (2014).
Firestone, A. J. et al. Small-molecule inhibitors of the AAA + ATPase motor cytoplasmic dynein. Nature 484, 125–129 (2012).
Senese, S. et al. Chemical dissection of the cell cycle: probes for cell biology and anti-cancer drug development. Cell. Death Dis. 5, e1462 (2014).
McNamara, D. E., Senese, S., Yeates, T. O. & Torres, J. Z. Structures of potent anticancer compounds bound to tubulin. Protein Sci. 24, 1164–1172 (2015).
Gholkar, A. A., Gimeno, T. V., Edgemon, J. E., Sim, M. S. & Torres, J. Z. MI-181 modulates cilia length and restores cilia length in cells with defective shortened cilia. ACS Chem. Biol. 19, 1733–1742 (2024).
Durra, A. et al. Unflavored electronic cigarette exposure induces alterations in airway ciliary structure and function. Respir Res. 26, 223 (2025).
Aufderheide, M., Scheffler, S., Ito, S., Ishikawa, S. & Emura, M. Ciliatoxicity in human primary bronchiolar epithelial cells after repeated exposure at the air-liquid interface with native mainstream smoke of K3R4F cigarettes with and without charcoal filter. Exp. Toxicol. Pathol. 67, 407–411 (2015).
Durra, A. et al. Protocol for constructing an accessible exposure chamber for in vitro and in vivo modeling of airway environmental exposures. STAR. Protoc. 6, 104069 (2025).
Thomas, J. et al. Transcriptional control of genes involved in ciliogenesis: a first step in making cilia. Biol. Cell. 102, 499–513 (2010).
Gomperts, B. N., Gong-Cooper, X. & Hackett, B. P. Foxj1 regulates basal body anchoring to the cytoskeleton of ciliated pulmonary epithelial cells. J. Cell. Sci. 117, 1329–1337 (2004).
Sharma, N., Kosan, Z. A., Stallworth, J. E., Berbari, N. F. & Yoder, B. K. Soluble levels of cytosolic tubulin regulate ciliary length control. Mol. Biol. Cell. 22, 806–816 (2011).
Jain, R. et al. Temporal relationship between primary and motile ciliogenesis in airway epithelial cells. Am. J. Respir Cell. Mol. Biol. 43, 731–739 (2010).
Simionescu, N. & Simionescu, M. Galloylglucoses of low molecular weight as mordant in electron microscopy. II. The moiety and functional groups possibly involved in the mordanting effect. J. Cell. Biol. 70, 622–633 (1976).
Ortug, C. Scanning electron microscopic findings in respiratory nasal mucosa following cigarette smoke exposure in rats. Ann. Anat. 185, 207–210 (2003).
Iskander, N. M., El-Hennawi, D. M., Yousef, T. F., El-Tabbakh, M. T. & Elnahriry, T. A. Evaluation of the effect of cigarette smoking on the olfactory neuroepithelium of new Zealand white rabbit, using scanning electron microscope. Eur. Arch. Otorhinolaryngol. 274, 2461–2468 (2017).
Purkayastha, A. et al. Direct exposure to SARS-CoV-2 and cigarette smoke increases infection severity and alters the stem Cell-Derived airway repair response. Cell. Stem Cell. 27, 869–875e864 (2020).
Hegab, A. E. et al. Aldehyde dehydrogenase activity enriches for proximal airway basal stem cells and promotes their proliferation. Stem Cells Dev. 23, 664–675 (2014).
Paul, M. K. et al. Dynamic changes in intracellular ROS levels regulate airway basal stem cell homeostasis through Nrf2-dependent Notch signaling. Cell. Stem Cell. 15, 199–214 (2014).
Acknowledgements
Fig. 1a was made with BioRender.
Funding
This work was supported by the National Institutes of Health grants R35GM139539 to J.Z.T; T32GM145388 to T.V.G; T32ES015457 to C.C; and R01CA208303, U01HL175451, and U01HL153000 to B.N.G. Work was also supported by grants from the Tobacco-related Disease Research Program HIPRA 29IP-0597 and HIPA T31IR1637, DoD PR202868, and Burroughs Wellcome Fund 1020030 to B.N.G. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
A.A.G., C.C., T.V.G., C.N., C.Z., B.N.G., and J.Z.T. initiated the project, designed experiments, wrote the manuscript, and analyzed results.
Corresponding author
Ethics declarations
Competing interests
MI-181 is the subject of patent number US 10,913,750 B; from The Regents of the University of California; authors: Jorge Torres, Robert Damoiseaux, Todd Yeates, Silvia Senese, Dan McNamara, and Yu-Chen Lo; related to its compositions and uses related thereto.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gholkar, A.A., Cherry, C., Gimeno, T.V. et al. MI-181 enhances ciliation and cilia length in a cigarette smoke exposed airway epithelial model. Sci Rep (2026). https://doi.org/10.1038/s41598-026-37296-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-37296-2