Abstract
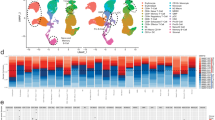
This cross-sectional study investigated the immunophenotype of patients with mucous membrane pemphigoid (MMP) and erosive oral lichen planus (eOLP) evaluating flow cytometry as additional method in differential diagnosis. Thirty patients (18 MMP and 12 eOLP) were recruited. A blood sample was collected from each patient and B and T lymphocytes subsets were characterized through flow cytometry. Disease severity was additionally scored and MMP patients were characterized as high or low-risk based on the extent of mucosal involvement. Any correlation was finally investigated between the immunophenotype and clinical and serological data. High-risk MMP reported higher frequencies of memory cytotoxic T cells (P < 0.01**), memory helper T cells (P < 0.001***), pre-switch and post-switch memory B cells (P < 0.01**) and lower frequencies of naïve cytotoxic T cells (P < 0.01**), naïve helper T cells (P < 0.001***) and naïve B cells (P < 0.05*) compared to low-risk MMP. Additionally, a significant lower frequency of Th1 cells (P < 0.05*) and memory cytotoxic T cells (P < 0.001***) was found in MMP compared to eOLP and both the biomarkers reported high diagnostic accuracy with an AU-ROC value of 0.76 (P < 0.05*) and 0.86 (P < 0.001***), respectively. No differences between the two diseases were reported in B-cell repertoire. T-cell immunophenotyping mirrors the different pathophysiology of MMP and eOLP and could provide a better understanding of the role of cellular immunity in MMP. MMP and eOLP can share similar clinical manifestations and can be challenging to distinguish through the histological and serological tests currently available. Flowcytometric assessment of T-cell subsets could be potentially useful in differentiating between the two diseases.
Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Schmidt, E. & Zillikens, D. Pemphigoid diseases. Lancet 381(9863), 320–332 (2013).
Schmidt, E. et al. Cicatricial pemphigoid: IgA and IgG autoantibodies target epitopes on both intra- and extracellular domains of bullous pemphigoid antigen 180. Br. J. Dermatol. 145(5), 778–783 (2001).
Oyama, N. et al. Bullous pemphigoid antigen II (BP180) and its soluble extracellular domains are major autoantigens in mucous membrane pemphigoid: the pathogenic relevance to HLA class II alleles and disease severity. Br. J. Dermatol. 154(1), 90–98 (2006).
Amber, K., Bloom, R. & Hertl, M. A systematic review with pooled analysis of clinical presentation and immunodiagnostic testing in mucous membrane pemphigoid: association of anti-laminin-332 IgG with oropharyngeal involvement and the usefulness of ELISA. J. Eur. Acad. Dermatol. Venereol. 30(1), 72–77 (2016).
Chan, L. S. et al. The first international consensus on mucous membrane pemphigoid: Definition, diagnostic criteria, pathogenic factors, medical treatment, and prognostic indicators. Arch. Dermatol. 138(3), 370–379 (2002).
Cizenski, J. D. et al. Spectrum of orocutaneous disease associations: Immune-mediated conditions. J. Am. Acad. Dermatol. 77(5), 795–806 (2017).
Du, G., Patzelt, S., Van Beek, N. & Schmidt, E. Mucous membrane pemphigoid. Autoimmun. rev. 21(4), 103036 (2022).
Leuci, S., Ruoppo, E., Adamo, D., Calabria, E. & Mignogna, M. D. Oral autoimmune vesicobullous diseases: Classification, clinical presentations, molecular mechanisms, diagnostic algorithms, and management. Periodontology 2000. 80(1), 77–88 (2019).
Rashid, H. et al. European guidelines (S3) on diagnosis and management of mucous membrane pemphigoid, initiated by the European Academy of Dermatology and Venereology – Part I. J. Eur. Acad. Dermatol. Venereol. 35(9), 1750–1764 (2021).
Carrozzo, M., Porter, S., Mercadante, V. & Fedele, S. Oral lichen planus: A disease or a spectrum of tissue reactions? Types, causes, diagnostic algorhythms, prognosis, management strategies. Periodontology 2000. 80(1), 105–125 (2019).
Alrashdan, M. S., Cirillo, N. & McCullough, M. Oral lichen planus: a literature review and update. Arch. Dermatol. Res. 308(8), 539–551 (2016).
Eisen, D., Carrozzo, M., Bagan Sebastian, J. V. & Thongprasom, K. Number V oral lichen planus: clinical features and management. Oral Dis. 11(6), 338–349 (2005).
Ismail, S. B., Kumar, S. K. S. & Zain, R. B. Oral lichen planus and lichenoid reactions: etiopathogenesis, diagnosis, management and malignant transformation. J. Oral Sci. 49(2), 89–106 (2007).
Helander, S. D. & Rogers, R. S. The sensitivity and specificity of direct Immunofluorescence testing in disorders of mucous membranes. J. Am. Acad. Dermatol. 30(1), 65–75 (1994).
Didona, D., Hinterseher, J. & Eming, R. Bullous autoimmune dermatoses of the mucous membranes. Dermatologie. 73(9), 692–700 (2022).
Carey, B. & Setterfield, J. Mucous membrane pemphigoid and oral blistering diseases. Clin. Exp. Dermatol. 44(7), 732–739 (2019).
Combemale, L. et al. Lichen planus pemphigoides with predominant mucous membrane involvement: a series of 12 patients and a literature review. Front. Immunol. 15, 1243566 (2024).
Solomon, L. W., Helm, T. N., Stevens, C., Neiders, M. E. & Kumar, V. Clinical and Immunopathologic findings in oral lichen planus pemphigoides. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 103(6), 808–813 (2007).
Barnadas, M. et al. Lichen planus pemphigoides: detection of anti-BP 180 antibodies by ELISA and immunoblotting tests. J. Eur. Acad. Dermatol. Venereol. 24(11), 1360–1361 (2010).
Fukuda, A. et al. Four cases of mucous membrane pemphigoid with clinical features of oral lichen planus. Int. J. Dermatol. 55(6), 657–665 (2016).
Bavarian, R., Sultan, A. S. & Mignogna, M. D. Comments regarding four cases of mucous membrane pemphigoid with clinical features of oral lichen planus and on the utility of immunofluorescence. Int. J. Dermatol. 56(12), 1515–1515 (2017).
Carrozzo, M. A reappraisal of diagnostic criteria for mucous membrane pemphigoid. J. Oral Pathol. Med. 38(1), 160 (2009).
Setterfield, J. et al. Mucous membrane pemphigoid: a dual circulating antibody response with IgG and IgA signifies a more severe and persistent disease. Br. J. Dermatol. 138(4), 602–610 (1998).
Shimanovich, I., Nitz, J. M. & Zillikens, D. Multiple and repeated sampling increases the sensitivity of direct immunofluorescence testing for the diagnosis of mucous membrane pemphigoid. J. Am. Acad. Dermatol. 77(4), 700–705e3 (2017).
Schmidt, E. et al. European guidelines (S3) on diagnosis and management of mucous membrane pemphigoid, initiated by the European Academy of Dermatology and Venereology – Part II. J. Eur. Acad. Dermatol. Venereol. 35(10), 1926–1948 (2021).
Carvajal Alegria, G., Gazeau, P., Hillion, S., Daïen, C. I. & Cornec, D. Y. K. Could lymphocyte profiling be useful to diagnose systemic autoimmune diseases? Clin. Rev. Allerg. Immunol. 53(2), 219–236 (2017).
Bergantini, L. et al. Effects of rituximab therapy on B cell differentiation and depletion. Clin. Rheumatol. 39(5), 1415–1421 (2020).
Preglej, T. et al. Advanced immunophenotyping: A powerful tool for immune profiling, drug screening, and a personalized treatment approach. Front. Immunol. 14 (2023).
Cheng, Y. S. L., Gould, A., Kurago, Z., Fantasia, J. & Muller, S. Diagnosis of oral lichen planus: a position paper of the American Academy of Oral and Maxillofacial Pathology. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 122(3), 332–354 (2016).
Escudier, M. et al. A scoring system for mucosal disease severity with special reference to oral lichen planus. Br. J. Dermatol. 157(4), 765–770 (2007).
Hofmann, S. C. et al. S2k guideline for the diagnosis and treatment of mucous membrane pemphigoid. J. Dtsch. Dermatol. Ges. 20(11), 1530–1550 (2022).
Sugerman, P. B. et al. The pathogenesis of oral lichen planus. Crit. Rev. Oral Biol. Med. 13(4), 350–365 (2002).
Kamaguchi, M. & Iwata, H. The diagnosis and blistering mechanisms of mucous membrane pemphigoid. Front. Immunol. 10, 34 (2019).
Scully, C. & Porter, S. R. The clinical spectrum of desquamative gingivitis. Semin Cutan. Med. Surg. 16(4), 308–313 (1997).
Mignogna, M. D. et al. Lichen planus pemphigoides, a possible example of epitope spreading. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 109(6), 837–843 (2010).
Warnakulasuriya, S. et al. Oral potentially malignant disorders: A consensus report from an international seminar on nomenclature and classification, convened by the WHO Collaborating Centre for Oral Cancer.. Oral Dis.. 27(8), 1862–1880 (2021).
González-Moles, M. Á. et al. Malignant transformation risk of oral lichen planus: A systematic review and comprehensive meta-analysis. Oral Oncol. 96, 121–130 (2019).
Rodríguez-Núñez, I., Blanco-Carrión, A., García, A. G. & Rey, J. G. Peripheral T-cell subsets in patients with reticular and atrophic-erosive oral lichen planus. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontics. 91(2), 180–188 (2001).
Charazinska-Carewicz, K., Ganowicz, E., Krol, M. & Gorska, R. Assessment of the peripheral immunocompetent cells in patients with reticular and atrophic-erosive lichen planus. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontics. 105(2), 202–205 (2008).
Wang, Y., Zhou, J., Fu, S., Wang, C. & Zhou, B. A study of association between oral lichen planus and immune balance of Th1/Th2 cells. Inflammation 38(5), 1874–1879 (2015).
Schinner, J. et al. Skin-infiltrating T cells display distinct inflammatory signatures in lichen planus, bullous pemphigoid and pemphigus vulgaris. Front. Immunol. 14, 1203776 (2023).
Bernauer, W., Wright, P., Dart, J. K., Leonard, J. N. & Lightman, S. The conjunctiva in acute and chronic mucous membrane pemphigoid. An immunohistochemical analysis. Ophthalmology 100(3), 339–346 (1993).
Elder, M. J. & Lightman, S. The immunological features and pathophysiology of ocular cicatricial pemphigoid. Eye. 8(Pt 2), 196–199 (1994).
Setterfield, J. et al. Mucous membrane pemphigoid: HLA-DQB1*0301 is associated with all clinical sites of involvement and May be linked to antibasement membrane IgG production. Br. J. Dermatol. 145(3), 406–414 (2001).
Carrozzo, M. et al. HLA-DQB1 alleles in Italian patients with mucous membrane pemphigoid predominantly affecting the oral cavity. Br. J. Dermatol. 145(5), 805–808 (2001).
Black, A. P. B. et al. Rapid effector function of circulating NC16A-specific T cells in individuals with mucous membrane pemphigoid. Br. J. Dermatol. 151(6), 1160–1164 (2004).
Canto-Gomes, J. et al. Low memory T cells blood counts and high Naïve regulatory T cells percentage at relapsing remitting multiple sclerosis diagnosis. Front. Immunol. 13, 901165 (2022).
Piantoni, S. et al. Effector T-cells are expanded in systemic lupus erythematosus patients with high disease activity and damage indexes. Lupus 27(1), 143–149 (2018).
Zou, Y. et al. The pathogenic role of CD4 + tissue-resident memory T cells bearing T follicular helper-like phenotype in pemphigus lesions. J. Invest. Dermatol. 141(9), 2141–2150 (2021).
Author information
Authors and Affiliations
Contributions
Author contribution: Simone Liguori: Conceptualization, Formal analysis, Investigation, Writing- Original draft, Dario Didona: Validation, Writing-Review & Editing, Elvira Ruoppo : Investigation, Resources, Antonia Fiore: Investigation, Writing- Original draft, Giulia Scalia: Methodology, Resources, Michele Davide Mignogna: Conceptualization, Supervision, Stefania Leuci: Methodology, Supervision, Writing-Review & Editing.
Corresponding author
Ethics declarations
Consent to participate
Written informed consent was provided by each patient taking part in this study.
Ethics approval
This study was conducted according to the World Medical Association Declaration of Helsinki. Approval was obtained by the Ethical Committee of the University of Naples Federico II (protocol number 69/19).
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liguori, S., Didona, D., Ruoppo, E. et al. Flow cytometry helps differentiate between mucous membrane pemphigoid and erosive oral lichen planus. Sci Rep (2026). https://doi.org/10.1038/s41598-026-37339-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-37339-8