Abstract
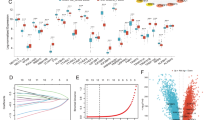
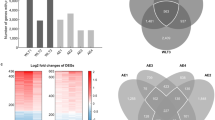
Chronic obstructive pulmonary disease (COPD) is characterized by chronic airway inflammation and is closely linked to oxidative stress. This study aimed to identify and validate key oxidative stress-related genes and pathways involved in COPD using integrated bioinformatics and experimental approaches. Public COPD datasets were obtained from the Gene Expression Omnibus (GEO) database, and oxidative stress-related genes were retrieved from the GeneCards database. Differentially expressed genes (DEGs) were screened and analyzed for functional enrichment. Machine-learning algorithms, including Least Absolute Shrinkage and Selection Operator (LASSO) regression and Random Forest, were used to identify hub genes and evaluate diagnostic value by calculating the area under the receiver operating characteristic (ROC) curve (AUC). Single-cell RNA sequencing (scRNA-seq) data were analyzed to determine the distribution of hub genes across different cell types. Finally, a COPD combined oxidative stress cell model was established using human bronchial epithelial cells (BEAS-2B), and key gene expression was experimentally validated. We identified 76 overlapping genes associated with both COPD and oxidative stress, mainly enriched in necroptosis, JAK-STAT, MAPK, and related pathways. 12 hub genes were screened using machine-learning methods. Single-cell analysis showed that TPPP3 and VEGFA were predominantly expressed in epithelial cells. Experimental validation confirmed the bioinformatics predictions at the gene level. This study identified and validated 12 oxidative stress-related hub genes in COPD, highlighting TPPP3 and VEGFA as key genes enriched in epithelial cells and potentially involved in tissue remodeling. These findings not only provide insights for exploring new therapeutic strategies but may also serve as potential diagnostic biomarkers or candidate therapeutic targets for COPD.
Similar content being viewed by others
Data availability
The datasets and codes used or analyzed during the current study are available from the corresponding author or first author upon request.
Abbreviations
- COPD:
-
Chronic obstructive pulmonary disease
- ROS:
-
Reactive oxygen species
- DEGs:
-
Differentially expressed genes
- FC:
-
Fold change
- GO:
-
Gene ontology
- KEGG:
-
Kyoto encyclopedia of genes and genomes
- LASSO:
-
Least absolute shrinkage and selection operator
- ROC:
-
Receiver operating characteristic
- AUC:
-
Area under the curve
- scRNA-seq:
-
Single-cell RNA sequencing
- PCA:
-
Principal component analysis
- t-SNE:
-
T-distributed stochastic neighbor embedding
- UMAP:
-
Uniform manifold approximation and projection
- BP:
-
Biological process
- CC:
-
Cellular component
- MF:
-
Molecular function
- CSE:
-
Cigarette smoke extract
- PBS:
-
Phosphate buffered saline
- DCFH-DA:
-
2',7'-Dichlorodihydrofluorescein diacetate
- SOD:
-
Superoxide dismutase
- CAT:
-
Catalase
- IL-1β:
-
Interleukin-1β
- IL-6:
-
Interleukin-6
- TNF-α:
-
Tumor necrosis factor-α
- ELISA:
-
Enzyme-linked immunosorbent assay
- JAK-STAT:
-
Janus kinase-signal transducer and activator of transcription
- qRT-PCR:
-
Quantitative real-time PCR
- cDNA:
-
Complementary DNA
- qPCR:
-
Quantitative polymerase chain reaction
- SEM:
-
Standard error of the mean
- MDG:
-
Mean decrease gini
- SVA:
-
Surrogate variable analysis
References
Wang, L. et al. The bidirectional gut-lung axis in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 207(9), 1145–1160 (2023).
López-Campos, J. L., Tan, W. & Soriano, J. B. Global burden of COPD. Respirology 21(1), 14–23 (2016).
Gordon, C. A., Madamanchi, N. R., Runge, M. S. & Jarstfer, M. B. Effect of oxidative stress on telomere maintenance in aortic smooth muscle cells. Biochim. Biophys. Acta Mol. Basis Dis. 1868(7), 166397 (2022).
Sies, H. Oxidative stress: a concept in redox biology and medicine. Redox Biol. 4, 180–183 (2015).
Barnes, P. J. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 138(1), 16–27 (2016).
Wang, C. et al. Progress in the mechanism and targeted drug therapy for COPD. Signal Transduct. Target Ther. 5(1), 248 (2020).
Kirkham, P. A. & Barnes, P. J. Oxidative stress in COPD. Chest 144(1), 266–273 (2013).
Nucera, F. et al. Role of oxidative stress in the pathogenesis of COPD. Minerva Med. 113(3), 370–404 (2022).
Barnes, P. J. Oxidative stress in chronic obstructive pulmonary disease. Antioxidants (Basel). 11(5), 965 (2022).
Repine, J. E., Bast, A. & Lankhorst, I. Oxidative stress in chronic obstructive pulmonary disease. oxidative stress study group. Am. J. Respir. Crit. Care Med. 156, 341–357 (1997).
Aghapour, M., Raee, P., Moghaddam, S. J., Hiemstra, P. S. & Heijink, I. H. Airway epithelial barrier dysfunction in chronic obstructive pulmonary disease: role of cigarette smoke exposure. Am. J. Respir. Cell Mol. Biol. 58(2), 157–169 (2018).
Aravamudan, B. et al. Cigarette smoke-induced mitochondrial fragmentation and dysfunction in human airway smooth muscle. Am. J. Physiol. Lung Cell Mol.Physiol. 306(9), L840-854 (2014).
Bowler, R. P., Barnes, P. J. & Crapo, J. D. The role of oxidative stress in chronic obstructive pulmonary disease. COPD 1(2), 255–277 (2004).
Papaconstantinou, J. The role of signaling pathways of inflammation and oxidative stress in development of senescence and aging phenotypes in cardiovascular disease. Cells 8(11), 1383 (2019).
Saunders, R. M., Biddle, M., Amrani, Y. & Brightling, C. E. Stressed out - The role of oxidative stress in airway smooth muscle dysfunction in asthma and COPD. Free Radic. Biol. Med. 185, 97–119 (2022).
Wang, Y., Huang, Z., Xiao, Y., Wan, W. & Yang, X. The shared biomarkers and pathways of systemic lupus erythematosus and metabolic syndrome analyzed by bioinformatics combining machine learning algorithm and single-cell sequencing analysis. Front. Immunol. 13, 1015882 (2022).
Rauschert, S., Raubenheimer, K., Melton, P. E. & Huang, R. C. Machine learning and clinical epigenetics: a review of challenges for diagnosis and classification. Clin. Epigenetics. 12(1), 51 (2020).
Sheikh, K., Coxson, H. O. & Parraga, G. This is what COPD looks like. Respirology 21(2), 224–236 (2016).
Lowe, K. E. et al. COPDGene(®) 2019: redefining the diagnosis of chronic obstructive pulmonary disease. Chronic Obstr. Pulm Dis. 6(5), 384–399 (2019).
Graham, B. L. et al. Standardization of spirometry 2019 update. an official american thoracic society and european respiratory society technical statement. Am. J. Respir. Crit. Care Med. 200(8), e70–e88 (2019).
Keener A. Redefining the diagnostic criteria for COPD. 2020; https://www.nature.com/articles/d41586-020-01373-x. Accessed May 2020.
Wu, T. et al. Clusterprofiler 4.0: A universal enrichment tool for interpreting omics data. Innovation (Camb). 2(3), 100141 (2021).
Yang, J., Liu, C., Li, L., Tu, X. & Lu, Z. Red blood cell distribution width predicts pulmonary hypertension secondary to chronic obstructive pulmonary disease. Can. Respir. J. 2019, 3853454 (2019).
Simon, N., Friedman, J., Hastie, T. & Tibshirani, R. Regularization paths for cox’s proportional hazards model via coordinate descent. J. Stat. Softw. 39(5), 1–13 (2011).
Gutor, S. S. et al. Characterization of immunopathology and small airway remodeling in constrictive bronchiolitis. Am. J. Respir. Crit. Care Med. 206(3), 260–270 (2022).
Jairaman, A. & Prakriya, M. Calcium signaling in airway epithelial cells: current understanding and implications for inflammatory airway disease. Arterioscler. Thromb. Vasc. Biol. 44(4), 772–783 (2024).
Shin, S. et al. Reduction of TRPC1/TRPC3 mediated Ca(2+)-signaling protects oxidative stress-induced COPD. Cell Signal. 107, 110681 (2023).
Liu, C. et al. Role of necroptosis in airflow limitation in chronic obstructive pulmonary disease: focus on small-airway disease and emphysema. Cell Death Discov. 8(1), 363 (2022).
Dera, A. A. et al. Thymoquinone (Tq) protects necroptosis induced by autophagy/mitophagy-dependent oxidative stress in human bronchial epithelial cells exposed to cigarette smoke extract (CSE). J. Food Biochem. 44(9), e13366 (2020).
Wen, X. et al. The PI3K/Akt-Nrf2 signaling pathway and mitophagy synergistically mediate hydroxytyrosol to alleviate intestinal oxidative damage. Int. J. Biol. Sci. 20(11), 4258–4276 (2024).
Zhang, B. et al. Arbutin attenuates LPS-induced acute kidney injury by inhibiting inflammation and apoptosis via the PI3K/Akt/Nrf2 pathway. Phytomedicine 82, 153466 (2021).
Li, J. et al. Hesperetin ameliorates hepatic oxidative stress and inflammation via the PI3K/AKT-Nrf2-ARE pathway in oleic acid-induced HepG2 cells and a rat model of high-fat diet-induced NAFLD. Food Funct. 12(9), 3898–3918 (2021).
Di Stefano, A. et al. Increased expression of nuclear factor-kappaB in bronchial biopsies from smokers and patients with COPD. Eur. Respir. J. 20(3), 556–563 (2002).
van der Toorn, M. et al. Lipid-soluble components in cigarette smoke induce mitochondrial production of reactive oxygen species in lung epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 297(1), L109-114 (2009).
Barnes, P. J. Oxidative stress-based therapeutics in COPD. Redox Biol. 33, 101544 (2020).
Scoditti, E., Massaro, M., Garbarino, S. & Toraldo, D. M. Role of diet in chronic obstructive pulmonary disease prevention and treatment. Nutrients 11(6), 1357 (2019).
Zhao, P. et al. Integration of transcriptomics, proteomics, metabolomics and systems pharmacology data to reveal the therapeutic mechanism underlying Chinese herbal Bufei Yishen formula for the treatment of chronic obstructive pulmonary disease. Mol. Med. Rep. 17(4), 5247–5257 (2018).
Sadowska, A. M. N-Acetylcysteine mucolysis in the management of chronic obstructive pulmonary disease. Ther. Adv. Respir. Dis. 6(3), 127–135 (2012).
Fan, X., Dong, T., Yan, K., Ci, X. & Peng, L. PM2.5 increases susceptibility to acute exacerbation of COPD via NOX4/Nrf2 redox imbalance-mediated mitophagy. Redox Biol. 59, 102587 (2023).
Xu, Y. et al. Isorhamnetin alleviates airway inflammation by regulating the Nrf2/Keap1 pathway in a mouse model of COPD. Front. Pharmacol. 13, 860362 (2022).
Liu, Y. et al. Progression of the PI3K/Akt signaling pathway in chronic obstructive pulmonary disease. Front. Pharmacol. 14, 1238782 (2023).
Zhu, L. et al. The antioxidant N-acetylcysteine promotes immune response and inhibits epithelial-mesenchymal transition to alleviate pulmonary fibrosis in chronic obstructive pulmonary disease by suppressing the VWF/p38 MAPK axis. Mol. Med. 27(1), 97 (2021).
Harvey, T., Flamenco, S. & Fan, C. M. A Tppp3(+)Pdgfra(+) tendon stem cell population contributes to regeneration and reveals a shared role for PDGF signalling in regeneration and fibrosis. Nat. Cell Biol. 21(12), 1490–1503 (2019).
Yea, J. H. et al. Tppp3(+) synovial/tendon sheath progenitor cells contribute to heterotopic bone after trauma. Bone Res. 11(1), 39 (2023).
White, A. L. & Bix, G. J. VEGFA isoforms as pro-angiogenic therapeutics for cerebrovascular diseases. Biomolecules 13(4), 702 (2023).
Wang, R. et al. MCPIP1 promotes cell proliferation, migration and angiogenesis of glioma via VEGFA-mediated ERK pathway. Exp Cell Res. 418(1), 113267 (2022).
Oláh, J., Lehotzky, A., Szénási, T., Berki, T. & Ovádi, J. Modulatory role of TPPP3 in microtubule organization and its impact on alpha-synuclein pathology. Cells 11(19), 1720 (2022).
Zhou, W., Li, J., Wang, X. & Hu, R. Stable knockdown of TPPP3 by RNA interference in Lewis lung carcinoma cell inhibits tumor growth and metastasis. Mol. Cell Biochem. 343(1–2), 231–238 (2010).
Li, Y. et al. Knockdown of tubulin polymerization promoting protein family member 3 suppresses proliferation and induces apoptosis in non-small-cell lung cancer. J. Cancer. 7(10), 1189–1196 (2016).
Poto, R. et al. Angiogenesis, lymphangiogenesis, and inflammation in chronic obstructive pulmonary disease (COPD): few certainties and many outstanding questions. Cells 11(10), 1720 (2022).
Claesson-Welsh, L. & Welsh, M. VEGFA and tumour angiogenesis. J. Intern Med. 273(2), 114–127 (2013).
Zhang, L. et al. CircRNA-miRNA-VEGFA: an important pathway to regulate cancer pathogenesis. Front. Pharmacol. 14, 1049742 (2023).
Patan, S. Vasculogenesis and angiogenesis as mechanisms of vascular network formation, growth and remodeling. J. Neurooncol. 50(1–2), 1–15 (2000).
McFee, R. M., Rozell, T. G. & Cupp, A. S. The balance of proangiogenic and antiangiogenic VEGFA isoforms regulate follicle development. Cell Tissue Res. 349(3), 635–647 (2012).
Lee, C. G. et al. Studies of vascular endothelial growth factor in asthma and chronic obstructive pulmonary disease. Proc Am Thorac Soc. 8(6), 512–515 (2011).
Ricciardolo, F. L. et al. Expression of vascular remodelling markers in relation to bradykinin receptors in asthma and COPD. Thorax 68(9), 803–811 (2013).
Guida, G. & Riccio, A. M. Immune induction of airway remodeling. Semin Immunol. 46, 101346 (2019).
Guan, R. et al. Hydrogen sulfide attenuates cigarette smoke-induced airway remodeling by upregulating SIRT1 signaling pathway. Redox Biol. 28, 101356 (2020).
Hirota, N. & Martin, J. G. Mechanisms of airway remodeling. Chest 144(3), 1026–1032 (2013).
Mannino, D. M. & Buist, A. S. Global burden of COPD: risk factors, prevalence, and future trends. Lancet 370(9589), 765–773 (2007).
Araya, J. et al. PRKN-regulated mitophagy and cellular senescence during COPD pathogenesis. Autophagy 15(3), 510–526 (2019).
Maremanda, K. P., Sundar, I. K. & Rahman, I. Role of inner mitochondrial protein OPA1 in mitochondrial dysfunction by tobacco smoking and in the pathogenesis of COPD. Redox Biol. 45, 102055 (2021).
Vij, N., Chandramani-Shivalingappa, P., Van Westphal, C., Hole, R. & Bodas, M. Cigarette smoke-induced autophagy impairment accelerates lung aging, COPD-emphysema exacerbations and pathogenesis. Am. J. Physiol.Cell Physiol. 314(1), C73-c87 (2018).
Funding
This study received financial support from the National Natural Science Foundation of China (No.81973631), The Science and Technology Commission of Shanghai Municipality (YDZX00003008), Basic research on kidney tonic formula for intervention in several airway inflammatory diseases (three projects)(2022QD056), 2023 Ground High Construction Project—First-class Integrative Medicine/Summit Discipline Construction—Cultivation of Provincial and Ministerial Key Laboratories of Integrative Medicine (DGF601013), and NDRC Equipment Reform Special Project-DK07 Basic Technology Platform for Scientific Research in the Disciplines of Integrative Medicine (DKF132001).
Author information
Authors and Affiliations
Contributions
Wenglam Choi conceived of the presented idea and performed the computations and, experimentation. Yueren Wu assisted with calculations and article writing as well as code handling. Wenjing Chen and Yuting Shi assisted in laboratory operations and data processing. Weihang Luo and Xinyuan Wu assisted experimental operation. Zhenhui Ruan collected references and data. Jingcheng Dong provided guidance and article correction. All authors discussed the results and contributed to the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Choi, W., Wu, Y., Chen, W. et al. Oxidative stress-associated genes TPPP3 and VEGFA in COPD revealed by bulk and single-cell sequencing analysis. Sci Rep (2026). https://doi.org/10.1038/s41598-026-37375-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-37375-4