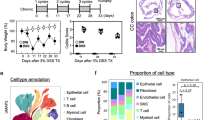
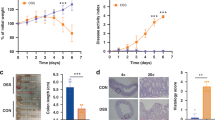
Abstract
Lanatoside C (LanaC), a cardiac glycoside, has been reported to possess therapeutic potential in acute intestinal inflammation; however, its in vivo effects on ulcerative colitis (UC) remain incompletely understood. In this study, we demonstrated that LanaC effectively attenuates DSS-induced colitis in mice by reducing inflammation, mitigating epithelial damage, and preserving barrier integrity. Mechanistically, LanaC treatment was associated with reduced macrophage infiltration in the colon and spleen, suppression of pro-inflammatory M1 macrophage markers, and enhancement of M2-associated markers. In vitro, LanaC inhibited LPS-induced M1 polarization and pro-inflammatory cytokine production in BMDMs, while promoting IL-4-driven M2 polarization and anti-inflammatory cytokine expression. These effects were accompanied by attenuation of STAT1/STAT3 signaling and enhancement of STAT6 activation, suggesting a selective reprogramming of macrophage responses. Collectively, these findings reveal that LanaC alleviates DSS-induced colitis, at least in part, through regulating macrophage infiltration and repolarization supporting its potential as a macrophage-targeted therapeutic candidate in UC.
Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study. The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Gros, B. & Kaplan, G. G. Ulcerative colitis in adults: A review. JAMA 330, 951–965. https://doi.org/10.1001/jama.2023.15389 (2023).
Du, L. & Ha, C. Epidemiology and pathogenesis of ulcerative colitis. Gastroenterol. Clin. North. Am. 49, 643–654. https://doi.org/10.1016/j.gtc.2020.07.005 (2020).
M’Koma, A. E. Inflammatory bowel disease: An expanding global health problem. Clin. Med. Insights Gastroenterol. 6, 33–47. https://doi.org/10.4137/CGast.S12731 (2013).
Awan, H., Fatima, U., Eaw, R., Knox, N. & Alrubaiy, L. The Efficacy of currently licensed biologics for treatment of ulcerative colitis: A literature review. Cureus 15, e37609. https://doi.org/10.7759/cureus.37609 (2023).
Bruscoli, S., Febo, M., Riccardi, C. & Migliorati, G. Glucocorticoid therapy in inflammatory bowel disease: Mechanisms and clinical practice. Front. Immunol. 12, 691480. https://doi.org/10.3389/fimmu.2021.691480 (2021).
Guo, R., Meng, Q., Wang, B. & Li, F. Anti-inflammatory effects of Platycodin D on dextran sulfate sodium (DSS) induced colitis and E. coli Lipopolysaccharide (LPS) induced inflammation. Int. Immunopharmacol. 94, 107474. https://doi.org/10.1016/j.intimp.2021.107474 (2021).
Ko, C. W. et al. AGA clinical practice guidelines on the management of mild-to-moderate ulcerative colitis. Gastroenterology 156, 748–764. https://doi.org/10.1053/j.gastro.2018.12.009 (2019).
Chen, S. et al. Macrophages in immunoregulation and therapeutics. Signal Transduct. Target Ther. 8, 207. https://doi.org/10.1038/s41392-023-01452-1 (2023).
Na, Y. R., Stakenborg, M., Seok, S. H. & Matteoli, G. Macrophages in intestinal inflammation and resolution: A potential therapeutic target in IBD. Nat. Rev. Gastroenterol. Hepatol. 16, 531–543. https://doi.org/10.1038/s41575-019-0172-4 (2019).
Luo, M., Zhao, F., Cheng, H., Su, M. & Wang, Y. Macrophage polarization: An important role in inflammatory diseases. Front. Immunol. 15, 1352946. https://doi.org/10.3389/fimmu.2024.1352946 (2024).
Moreira Lopes, T. C., Mosser, D. M. & Goncalves, R. Macrophage polarization in intestinal inflammation and gut homeostasis. Inflamm. Res. 69, 1163–1172. https://doi.org/10.1007/s00011-020-01398-y (2020).
Chen, Y. H. et al. Regorafenib enhances M1/M2 macrophage polarization by inhibiting the secretion of plasminogen activator inhibitor-1 in head and neck squamous cell carcinoma. Life Sci. 358, 123147. https://doi.org/10.1016/j.lfs.2024.123147 (2024).
Xiao, Q. et al. Formononetin alleviates ulcerative colitis via reshaping the balance of M1/M2 macrophage polarization in a gut microbiota-dependent manner. Phytomedicine 135, 156153. https://doi.org/10.1016/j.phymed.2024.156153 (2024).
Orecchioni, M., Ghosheh, Y., Pramod, A. B. & Ley, K. Corrigendum: Macrophage polarization: Different gene signatures in M1(LPS+) vs. classically and M2(LPS-) vs. alternatively activated macrophages. Front. Immunol. 11, 234. https://doi.org/10.3389/fimmu.2020.00234 (2020).
Ley, K., Pramod, A. B., Croft, M., Ravichandran, K. S. & Ting, J. P. How mouse macrophages sense what is going on. Front. Immunol. 7, 204. https://doi.org/10.3389/fimmu.2016.00204 (2016).
Han, X., Ding, S., Jiang, H. & Liu, G. Roles of macrophages in the development and treatment of gut inflammation. Front. Cell. Dev. Biol. 9, 625423. https://doi.org/10.3389/fcell.2021.625423 (2021).
Zhang, K., Guo, J., Yan, W. & Xu, L. Macrophage polarization in inflammatory bowel disease. Cell. Commun. Signal. 21, 367. https://doi.org/10.1186/s12964-023-01386-9 (2023).
Wu, M. M. et al. Dioscin ameliorates murine ulcerative colitis by regulating macrophage polarization. Pharmacol. Res. 172, 105796. https://doi.org/10.1016/j.phrs.2021.105796 (2021).
Canton, M. et al. Reactive oxygen species in macrophages: sources and targets. Front. Immunol. 12, 734229. https://doi.org/10.3389/fimmu.2021.734229 (2021).
Formentini, L. et al. Mitochondrial ROS production protects the intestine from inflammation through functional M2 macrophage polarization. Cell. Rep. 19, 1202–1213. https://doi.org/10.1016/j.celrep.2017.04.036 (2017).
Ito, R. et al. Involvement of IL-17A in the pathogenesis of DSS-induced colitis in mice. Biochem. Biophys. Res. Commun. 377, 12–16. https://doi.org/10.1016/j.bbrc.2008.09.019 (2008).
Botelho, A. F. M., Pierezan, F., Soto-Blanco, B. & Melo, M. M. A review of cardiac glycosides: Structure, toxicokinetics, clinical signs, diagnosis and antineoplastic potential. Toxicon 158, 63–68. https://doi.org/10.1016/j.toxicon.2018.11.429 (2019).
Ponce, A., Flores-Maldonado, C. & Contreras, R. G. Cardiac glycosides: From natural defense molecules to emerging therapeutic agents. Biomolecules https://doi.org/10.3390/biom150608854 (2025).
Chao, M. W. et al. Lanatoside C, a cardiac glycoside, acts through protein kinase Cdelta to cause apoptosis of human hepatocellular carcinoma cells. Sci. Rep. 7, 46134. https://doi.org/10.1038/srep46134 (2017).
Liu, X. & Lv, K. Cruciferous vegetables intake is inversely associated with risk of breast cancer: A meta-analysis. Breast 22, 309–313. https://doi.org/10.1016/j.breast.2012.07.013 (2013).
Jansson, D. et al. Cardiac glycosides target barrier inflammation of the vasculature, meninges and choroid plexus. Commun. Biol. 4, 260. https://doi.org/10.1038/s42003-021-01787-x (2021).
Zhu, W., Zhang, Z. & Wang, X. Network pharmacology analysis of Lanatoside C: Molecular targets and mechanisms in the treatment of ulcerative colitis. Front. Mol. Biosci. 12, 1552360. https://doi.org/10.3389/fmolb.2025.1552360 (2025).
Wirtz, S. et al. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat. Protoc. 12, 1295–1309. https://doi.org/10.1038/nprot.2017.044 (2017).
Morampudi, V. et al. The goblet cell-derived mediator RELM-beta drives spontaneous colitis in Muc2-deficient mice by promoting commensal microbial dysbiosis. Mucosal. Immunol. 9, 1218–1233. https://doi.org/10.1038/mi.2015.140 (2016).
Gonlubol, F., Siegel, A. & Bing, R. J. Effect of a cardiac glycoside (cedilanid) on the sodium and potassium balance of the human heart. Circ. Res. 4, 298–301. https://doi.org/10.1161/01.res.4.3.298 (1956).
Kerneur, C., Cano, C. E. & Olive, D. Major pathways involved in macrophage polarization in cancer. Front. Immunol. 13, 1026954. https://doi.org/10.3389/fimmu.2022.1026954 (2022).
Liu, T., Zhang, L., Joo, D. & Sun, S. C. NF-kappaB signaling in inflammation. Signal Transduct. Target. Ther. 2, 17023. https://doi.org/10.1038/sigtrans.2017.23 (2017).
Ohmori, Y. & Hamilton, T. A. Requirement for STAT1 in LPS-induced gene expression in macrophages. J. Leukoc. Biol. 69, 598–604 (2001).
Balic, J. J. et al. STAT3 serine phosphorylation is required for TLR4 metabolic reprogramming and IL-1beta expression. Nat. Commun. 11, 3816. https://doi.org/10.1038/s41467-020-17669-5 (2020).
Takeda, K. et al. Essential role of Stat6 in IL-4 signalling. Nature 380, 627–630. https://doi.org/10.1038/380627a0 (1996).
Kobayashi, T. et al. Ulcerative colitis. Nat. Rev. Dis. Primers 6, 74. https://doi.org/10.1038/s41572-020-0205-x (2020).
Solitano, V. et al. Reaching the therapeutic ceiling in IBD: Can advanced combination treatment (ACT) offer a solution?. Best Pract. Res. Clin. Gastroenterol. https://doi.org/10.1016/j.bpg.2025.101981 (2025).
Matar, A., Damianos, J. A., Jencks, K. J. & Camilleri, M. Intestinal barrier impairment, preservation, and repair: An update. Nutrients https://doi.org/10.3390/nu16203494 (2024).
Yang, W. H. et al. Innate mechanism of mucosal barrier erosion in the pathogenesis of acquired colitis. iScience 26, 107883. https://doi.org/10.1016/j.isci.2023.107883 (2023).
Antoni, L., Nuding, S., Wehkamp, J. & Stange, E. F. Intestinal barrier in inflammatory bowel disease. World J. Gastroenterol. 20, 1165–1179. https://doi.org/10.3748/wjg.v20.i5.1165 (2014).
Neurath, M. F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 14, 329–342. https://doi.org/10.1038/nri3661 (2014).
Neurath, M. F., Artis, D. & Becker, C. The intestinal barrier: a pivotal role in health, inflammation, and cancer. Lancet Gastroenterol. Hepatol. 10, 573–592. https://doi.org/10.1016/S2468-1253(24)00390-X (2025).
Bain, C. C. & Schridde, A. Origin, differentiation, and function of intestinal macrophages. Front. Immunol. 9, 2733. https://doi.org/10.3389/fimmu.2018.02733 (2018).
Lissner, D. et al. Monocyte and M1 macrophage-induced barrier defect contributes to chronic intestinal inflammation in IBD. Inflamm. Bowel Dis. 21, 1297–1305. https://doi.org/10.1097/MIB.0000000000000384 (2015).
Zhou, X. et al. YAP aggravates inflammatory bowel disease by regulating M1/M2 macrophage polarization and gut microbial homeostasis. Cell. Rep. 27, 1176–1189. https://doi.org/10.1016/j.celrep.2019.03.028 (2019).
Zhang, J. et al. Na/K-ATPase suppresses LPS-induced pro-inflammatory signaling through Lyn. iScience 25, 104963. https://doi.org/10.1016/j.isci.2022.104963 (2022).
Chen, Y. et al. Cardiotonic steroids stimulate macrophage inflammatory responses through a pathway involving CD36, TLR4, and Na/K-ATPase. Arterioscler. Thromb. Vasc. Biol. 37, 1462–1469. https://doi.org/10.1161/ATVBAHA.117.309444 (2017).
Li, Z. et al. Depletion of tumor associated macrophages enhances local and systemic platelet-mediated anti-PD-1 delivery for post-surgery tumor recurrence treatment. Nat. Commun. 13, 1845. https://doi.org/10.1038/s41467-022-29388-0 (2022).
Kang, D. S. et al. Macrophage transfer promotes intestinal mucosal healing by encouraging transit-amplifying cell expansion in mice. Front. Immunol. 16, 1555695. https://doi.org/10.3389/fimmu.2025.1555695 (2025).
Watanabe, H., Numata, K., Ito, T., Takagi, K. & Matsukawa, A. Innate immune response in Th1- and Th2-dominant mouse strains. Shock 22, 460–466. https://doi.org/10.1097/01.shk.0000142249.08135.e9 (2004).
Hartmann, W., Blankenhaus, B., Brunn, M. L., Meiners, J. & Breloer, M. Elucidating different pattern of immunoregulation in BALB/c and C57BL/6 mice and their F1 progeny. Sci. Rep. 11, 1536. https://doi.org/10.1038/s41598-020-79477-7 (2021).
Nakase, H., Sato, N., Mizuno, N. & Ikawa, Y. The influence of cytokines on the complex pathology of ulcerative colitis. Autoimmun. Rev. 21, 103017. https://doi.org/10.1016/j.autrev.2021.103017 (2022).
Yang, C. & Merlin, D. Unveiling colitis: A journey through the dextran sodium sulfate-induced model. Inflamm. Bowel Dis. 30, 844–853. https://doi.org/10.1093/ibd/izad312 (2024).
Bamias, G. & Cominelli, F. Role of type 2 immunity in intestinal inflammation. Curr. Opin. Gastroenterol. 31, 471–476. https://doi.org/10.1097/MOG.0000000000000212 (2015).
Yang, F. et al. Th1/Th2 balance and Th17/Treg-mediated immunity in relation to murine resistance to dextran sulfate-induced colitis. J. Immunol. Res. 2017, 7047201. https://doi.org/10.1155/2017/7047201 (2017).
Chandwaskar, R. et al. Dysregulation of T cell response in the pathogenesis of inflammatory bowel disease. Scand. J. Immunol. 100, e13412. https://doi.org/10.1111/sji.13412 (2024).
Funding
This study was supported by the National Natural Science Foundation of China (T2341019), NSFC Guangdong Joint Fund (U1132001), General Program of the National Natural Science Foundation of China (82174243 and 82204948), Natural Science Foundation of Guangdong Province, China (2023A1515110757), Guangzhou Science and Technology Plan Project (2024B03J1343), Major Sci-entific and Technological Project of Guangzhou Municipal Health Commission (20252D003), Re-search Project of Traditional Chinese Medicine Bureau of Guangdong Province (20241208), Gen-eral project of Beijing Natural Science Foundation (7242227), Fundamental Research Funds for the Central Universities (BZY-JMZY-2022-001 and 2023-JYB-JBZD-009), High-level Key Discipline of the National Administration of Traditional Chinese Medicine-Traditional Chinese Constitutional Medicine (zyyzdxk-2023251), Major Science and Technology Special Projects in Hubei Province (2023BCA005), and the Chief Scientist Research Project of Hubei Shizhen Laboratory (HSL2024SX0002).
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design of the study. LY and HZ conceived and designed the research. LY, HZ, and JL performed all experiments. LY and JL analyzed the data and prepared the figures. LY, HZ, and JW interpreted the experimental results and drafted the manuscript. XZ and JW were responsible for project administration and funding acquisition. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yu, L., Liu, J., Zhao, X. et al. Lanatoside C ameliorates DSS-induced colitis with improved intestinal barrier integrity and reduced M1 macrophage polarization. Sci Rep (2026). https://doi.org/10.1038/s41598-026-37484-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-37484-0