Abstract
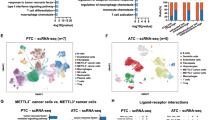
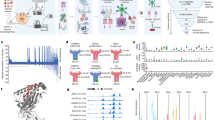
Intra-tumor nerve infiltration has been correlated with poor prognosis of tumor, particularly in head and neck squamous cell carcinoma (HNSCC). While emerging evidence highlights the critical role of neuroimmune crosstalk in tumor progression and immunosuppression, the molecular mediators of this axis remain poorly understood. This study has identified cancer cell-secreted PTHLH/PTHrP as a key regulator of this neuroimmune network through integrated transcriptomic analysis, CRISPR-mediated knockout, and parallel preclinical models in immunodeficient (BALB/c nude) and immunocompetent (C57BL/6J) mice. Our finding revealed significant higher expression of PTHLH correlated with neuroimmune factors and poor prognosis in immunotherapy outcome. Genetic ablation of PTHLH significantly altered the expression of neurotrophic factors essential for intra-tumoral nerve growth. Strikingly, while PTHLH knockout showed no significant anti-tumor response in vitro and in immunodeficient mice, it dramatically reduced tumor burden in immunocompetent hosts. Comprehensive immune profiling further demonstrated enhanced immunoreactivity (increased CD8 + and CD4 + T cells) alongside reduced immunosuppression (decreased FOXP3 + Tregs and PD-L1 expression) in PTHLH-deficient tumors. Moreover, these tumors also showed lower expression of tumor proliferative and neuron markers. Together these findings established cancer cell secreted PTHLH as a critical mediator of immunosuppression and neuron infiltrations in HNSCC, particularly in tongue tumor.
Similar content being viewed by others
Data availability
The data supporting this study are available from the corresponding author upon reasonable request.
References
Chen, F. et al. Pan-Cancer molecular classes transcending tumor lineage across 32 cancer Types, multiple data Platforms, and over 10,000 cases. Clin. Cancer Res. 24, 2182–2193. https://doi.org/10.1158/1078-0432.CCR-17-3378 (2018).
Schmitd, L. B., Scanlon, C. S. & D’Silva, N. J. Perineural invasion in head and neck cancer. J. Dent. Res. 97, 742–750. https://doi.org/10.1177/0022034518756297 (2018).
Lee, T. L. et al. Nerve-tumour interaction enhances the aggressiveness of oral squamous cell carcinoma. Clin. Otolaryngol. 44, 1087–1095. https://doi.org/10.1111/coa.13452 (2019).
Amit, M. et al. Loss of p53 drives neuron reprogramming in head and neck cancer. Nature 578, 449–454. https://doi.org/10.1038/s41586-020-1996-3 (2020).
Case, A. J. & Zimmerman, M. C. Sympathetic-mediated activation versus suppression of the immune system: Consequences for hypertension. J. Physiol. 594, 527–536. https://doi.org/10.1113/JP271516 (2016).
Cutz, E., Chan, W., Track, N. S., Goth, A. & Said, S. I. Release of vasoactive intestinal polypeptide in mast cells by Histamine liberators. Nature 275, 661–662. https://doi.org/10.1038/275661a0 (1978).
Gonzalez-Rey, E., Chorny, A. & Delgado, M. Regulation of immune tolerance by anti-inflammatory neuropeptides. Nat. Rev. Immunol. 7, 52–63. (2007).
Balood, M. et al. Nociceptor neurons affect cancer immunosurveillance. Nature 611, 405–412 (2022).
Armaiz-Pena, G. N. et al. Adrenergic regulation of monocyte chemotactic protein 1 leads to enhanced macrophage recruitment and ovarian carcinoma growth. Oncotarget 6, 4266–4273. https://doi.org/10.18632/oncotarget.2887 (2015).
Rosas-Ballina, M. et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 334, 98–101. https://doi.org/10.1126/science.1209985 (2011).
Ali, S., Yaniv, D. & Amit, M. Neuroimmune interactions and their role in carcinogenesis. In: (eds Amit, M. & Scheff, N. N.) Cancer Neuroscience. Cham: Springer International Publishing; 83–99. (2023).
Edwards, C. M. & Johnson, R. W. From good to bad: The opposing effects of PTHrP on tumor growth, dormancy, and metastasis throughout cancer progression. Front. Oncol. 11, 644303. https://doi.org/10.3389/fonc.2021.644303 (2021).
Goldner, W., Cancer-related & hypercalcemia J. Oncol. Pract. 12, 426 – 32. https://doi.org/10.1200/JOP.2016.011155 (2016).
Mundy, G. R. & Edwards, J. R. PTH-related peptide (PTHrP) in hypercalcemia. J. Am. Soc. Nephrol. 19, 672–675. https://doi.org/10.1681/ASN.2007090981 (2008).
Mundy, G. R. Metastasis to bone: Causes, consequences and therapeutic opportunities. Nat. Rev. Cancer. 2, 584–593. https://doi.org/10.1038/nrc867 (2002).
Garcia, M. et al. Parathyroid hormone-like hormone plays a dual role in neuroblastoma depending on PTH1R expression. Mol. Oncol. 13, 1959–1975. https://doi.org/10.1002/1878-0261.12542 (2019).
Almadori, G. et al. Parathyroid hormone-related peptide and parathyroid hormone-related peptide receptor type 1 in locally advanced laryngeal cancer as prognostic indicators of relapse and survival. BMC Cancer. 22, 704. https://doi.org/10.1186/s12885-022-09748-1 (2022).
Shalaby, M., Dawoud, M. M., Gadallah, M. S. & Abdou, A. G. Immunohistochemical expression of parathyroid hormone-related protein and Ezrin in invasive breast carcinoma of no special type: A retrospective analysis. Diagn. Pathol. 20, 8. https://doi.org/10.1186/s13000-025-01598-2 (2025).
Martin, T. J. & Johnson, R. W. Multiple actions of parathyroid hormone-related protein in breast cancer bone metastasis. Br. J. Pharmacol. 178, 1923–1935. https://doi.org/10.1111/bph.14709 (2021).
Li, J. et al. PTHrP drives breast tumor initiation, progression, and metastasis in mice and is a potential therapy target. J. Clin. Invest. 121, 4655–4669. https://doi.org/10.1172/JCI46134 (2011).
Pitarresi, J. R. et al. PTHrP drives pancreatic cancer growth and metastasis and reveals a new therapeutic vulnerability. Cancer Discov. 11, 1774–1791. https://doi.org/10.1158/2159-8290.CD-20-1098 (2021).
Chang, W. M. et al. Parathyroid hormone-Like hormone is a poor prognosis marker of head and neck cancer and promotes cell growth via RUNX2 regulation. Sci. Rep. 7, 41131. https://doi.org/10.1038/srep41131 (2017).
Gu, Z. et al. Absence of PTHrP nuclear localization and carboxyl terminus sequences leads to abnormal brain development and function. PLoS One. 7, e41542 (2012).
Fukayama, S., Tashjian, A. H. Jr, Davis, J. N. & Chisholm, J. C. Signaling by N-and C-terminal sequences of parathyroid hormone-related protein in hippocampal neurons. Proc. Natl. Acad. Sci. 92, 10182–10186 (1995).
Shepherd, A., Mickle, A., McIlvried, L., Gereau, I. V. R. & Mohapatra, D. Parathyroid hormone-related peptide activates and modulates TRPV 1 channel in human DRG neurons. Eur. J. Pain. 22, 1685–1690 (2018).
Tang, Z., Kang, B., Li, C., Chen, T. & Zhang, Z. GEPIA2: An enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 47, W556–W60. https://doi.org/10.1093/nar/gkz430 (2019).
Li, T. et al. TIMER: A web server for comprehensive analysis of Tumor-Infiltrating immune cells. Cancer Res. 77, e108–e10. https://doi.org/10.1158/0008-5472.CAN-17-0307 (2017).
Győrffy, B. Integrated analysis of public datasets for the discovery and validation of survival-associated genes in solid tumors. Innovation. 5. (2024).
Ayala, G. E. et al. Cancer-related axonogenesis and neurogenesis in prostate cancer. Clin. Cancer Res. 14, 7593–7603. https://doi.org/10.1158/1078-0432.CCR-08-1164 (2008).
Silverman, D. A. et al. Cancer-associated neurogenesis and nerve-cancer cross-talk. Cancer Res. 81, 1431–1440. https://doi.org/10.1158/0008-5472.CAN-20-2793 (2021).
Vermeer, P. D., Restaino, A. C., Barr, J. L., Yaniv, D. & Amit, M. Nerves at play: The peripheral nervous system in extracranial malignancies. Cancer Discov. 15, 52–68. https://doi.org/10.1158/2159-8290.CD-23-0397 (2025).
Meyers, E. A. & Kessler, J. A. TGF-beta family signaling in neural and neuronal differentiation, development, and function. Cold Spring Harb Perspect. Biol. https://doi.org/10.1101/cshperspect.a022244 (2017).
Godinho-Silva, C., Cardoso, F. & Veiga-Fernandes, H. Neuro-Immune cell units: A new paradigm in physiology. Annu. Rev. Immunol. 37, 19–46. https://doi.org/10.1146/annurev-immunol-042718-041812 (2019).
Gentles, A. J. et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat. Med. 21, 938–945. https://doi.org/10.1038/nm.3909 (2015).
Yuen, G. J., Demissie, E. & Pillai, S. B lymphocytes and cancer: A love-hate relationship. Trends Cancer. 2 https://doi.org/10.1016/j.trecan.2016.10.010 (2016). :747 – 57.
Renz, B. W. et al. β2 adrenergic-neurotrophin feedforward loop promotes pancreatic cancer. Cancer cell. 33, 75–90 (2018). e7.
Wang, K. et al. The neurotrophic factor neurturin contributes toward an aggressive cancer cell phenotype, neuropathic pain and neuronal plasticity in pancreatic cancer. Carcinogenesis 35, 103–113. https://doi.org/10.1093/carcin/bgt312 (2014).
Ding, Y. et al. Semaphorin 4F as a critical regulator of neuroepithelial interactions and a biomarker of aggressive prostate cancer. Clin. Cancer Res. 19, 6101–6111. https://doi.org/10.1158/1078-0432.CCR-12-3669 (2013).
Kuske, M., Haist, M., Jung, T., Grabbe, S. & Bros, M. Immunomodulatory properties of immune checkpoint Inhibitors-More than boosting T-Cell responses? Cancers (Basel). 14. https://doi.org/10.3390/cancers14071710 (2022).
Wang, D. R., Wu, X. L. & Sun, Y. L. Therapeutic targets and biomarkers of tumor immunotherapy: Response versus non-response. Signal. Transduct. Target. Ther. 7, 331. https://doi.org/10.1038/s41392-022-01136-2 (2022).
Gonzalez-Tafoya, E. et al. TNF contributes to T-cell exhaustion in chronic L. mexicana infections of mice through PD-L1 up-regulation. Cell. Immunol. 358, 104196. https://doi.org/10.1016/j.cellimm.2020.104196 (2020).
Yang, W. F., Wong, M. C. M., Thomson, P. J., Li, K. Y. & Su, Y. X. The prognostic role of PD-L1 expression for survival in head and neck squamous cell carcinoma: A systematic review and meta-analysis. Oral Oncol. 86, 81–90. https://doi.org/10.1016/j.oraloncology.2018.09.016 (2018).
Letterio, J. J. & Roberts, A. B. Regulation of immune responses by TGF-beta. Annu. Rev. Immunol. 16, 137–161. https://doi.org/10.1146/annurev.immunol.16.1.137 (1998).
Funding
No funding was obtained for this study.
Author information
Authors and Affiliations
Contributions
R.K.: Conceptualization, Data curation, formal analysis, investigation, methodology, project administration, visualization, writing-original draft; G.Z.: Formal analysis, writing-review and editing, W.Y.: Conceptualization, formal analysis, methodology, project administration, validation, supervision, writing-review and editing, Y.S.: Conceptualization, methodology, project administration, formal analysis, supervision, funding acquisition, resources, writing–review and editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kishan, R., Zhang, G., Yang, W. et al. Ablation of cancer cell secreted neuropeptide PTHLH/PTHrP provokes anti-tumor immunity in murine tongue squamous cell carcinoma. Sci Rep (2026). https://doi.org/10.1038/s41598-026-37580-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-37580-1