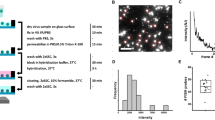
Abstract
This study demonstrates that high-frequency ultrasound (3–20 MHz) can effectively disrupt the structural integrity of both Influenza A virus (H1N1) and SARS-CoV-2 through a resonance-driven mechanism distinct from classical cavitation (kHz range). Under these conditions, viral particles undergo pronounced alterations (fragmentation, envelope rupture, and loss of morphological uniformity) consistent with direct mechanical destabilization rather than thermal or bubble-mediated effects. Detailed structural analyses revealed significant disruption of the viral envelope, accompanied by measurable shifts in particle size distribution and reduced diameters, indicative of resonance-induced fragmentation. These structural modifications were paralleled by biological consequences: SARS-CoV-2 infectivity was markedly reduced in vitro, with infected cells exhibiting substantially lower viral loads. Importantly, this work provides the first experimental evidence that acoustic resonance can directly couple with viral structural components, inducing selective mechanical destabilization of the envelope. The convergence of structural and functional data supports the view that this represents a previously undescribed biophysical phenomenon, fundamentally distinct from cavitation. This resonance-mediated destabilization highlights a novel, non-invasive, and broad-spectrum antiviral strategy that differs from cavitation, more suited to asepsis and sterilization, and offers a therapeutic approach with potential applications against enveloped respiratory viruses and other clinically relevant pathogens.
Similar content being viewed by others
Data availability
Data supporting this study are available from the corresponding author upon reasonable request. When applicable, processed datasets and code are available in public repositories as described in Methods.
References
Rastogi, R. P., Richa Kumar, A., Tyagi, M. B. & Sinha, R. P. Molecular mechanisms of ultraviolet radiation-induced DNA damage and repair. J. Nucleic Acids 2010, 592980. https://doi.org/10.4061/2010/592980 (2010).
Norval, M. The effect of ultraviolet radiation on human viral infections. Photochem. Photobiol. 82, 1495. https://doi.org/10.1562/2006-07-28-IR-987 (2006).
Tuieng, R. J., Cartmell, S. H., Kirwan, C. C. & Sherratt, M. J. The effects of ionising and non-ionising electromagnetic radiation on extracellular matrix proteins. Cells 10, 3041. https://doi.org/10.3390/cells10113041 (2021).
Tiong, T. J., Chu, J. K. & Tan, K. W. Advancements in acoustic cavitation modelling: progress, challenges, and future directions in sonochemical reactor design. Ultrason. Sonochem. 112, 107163. https://doi.org/10.1016/j.ultsonch.2024.107163 (2025).
Wierzbicki, T., Li, W., Liu, Y. & Zhu, J. Effect of receptors on the resonant and transient harmonic vibrations of coronavirus. J. Mech. Phys. Solids 150, 104369. https://doi.org/10.1016/j.jmps.2021.104369 (2021).
Kausar, S. et al. Mechanism of action of antiviral drugs: a review. Int. J. Immunopathol. Pharmacol. 35, 20587384211002620. https://doi.org/10.1177/20587384211002621 (2021).
Chaudhuri, S., Symons, J. A. & Deval, J. Innovation and trends in the development and approval of antiviral medicines: 1987–2017 and beyond. Antivir. Res. 155, 76–88. https://doi.org/10.1016/j.antiviral.2018.05.005 (2018).
Mink, M. et al. Impact of HIV-1 gp41 amino acid substitutions selected during enfuvirtide treatment on binding and antiviral potency. J. Virol. 79, 12447–12454. https://doi.org/10.1128/JVI.79.19.12447-12454.2005 (2005).
Sheahan, T. P. et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 9, eaal3653. https://doi.org/10.1126/scitranslmed.aal3653 (2017).
Qiu, S. et al. Binding mode of fusion inhibitor T20 onto HIV-1 gp41 and mechanisms of resistance explored by computational study. Curr. HIV Res. 10, 182–194. https://doi.org/10.2174/157016212799937191 (2012).
Beigel, J. H. et al. Remdesivir for the treatment of COVID-19 – final report. N. Engl. J. Med. 383, 1813–1826. https://doi.org/10.1056/NEJMoa2007764 (2020).
Wu, W. et al. The SARS-CoV-2 nucleocapsid protein: role in viral life cycle, structure and functions, and use as a potential target. Virol. J. 20, 6. https://doi.org/10.1186/s12985-023-01968-6 (2023).
Ghoshal, G., Luchies, A., Blue, J. & Oelze, M. Temperature-dependent ultrasonic characterization of biological media. J. Acoust. Soc. Am. 130, 2203. https://doi.org/10.1121/1.3626162 (2011).
Viet, N. V., Karathanasopoulos, N. & Zaki, W. Mechanical attributes and wave propagation of TPMS lattice structures. Mech. Mater. 172, 104363. https://doi.org/10.1016/j.mechmat.2022.104363 (2022).
Schaap, A., Eghiaia, F., George, A. D. & Veigel, C. Effect of envelope proteins on mechanical properties of influenza virus. J. Biol. Chem. 287, 41078–41088. https://doi.org/10.1074/jbc.M112.412726 (2012).
Mariangeli, M. et al. Mechanical properties of SARS-CoV-2 particles and effects of photosensitizer hypericin. Int. J. Mol. Sci. 25, 68724. https://doi.org/10.3390/ijms25168724 (2024).
Nonn, B. et al. Mechanical properties of SARS-CoV-2 inferred by nano-indentation and simulations. J. Mech. Behav. Biomed. Mater. 148, 106153. https://doi.org/10.1016/j.jmbbm.2023.106153 (2023).
Zhang, Y. et al. Nanoscopic acoustic vibrational dynamics of a single virus captured by ultrafast spectroscopy. Proc. Natl Acad. Sci. USA 122, e2420428122 (2025).
Feroz, N. Brownian motions and thermophoresis effects in a vertically stretching sheet with micropolar nanofluid flow. Mod. Phys. Lett. B 38, 2450059 (2024).
Martinez-Martin, D. et al. Resolving structure and mechanical properties at the nanoscale of viruses with frequency modulation atomic force microscopy. PLoS ONE 7, e30204 (2012).
Michel, J. P. et al. Nanoindentation studies of full and empty viral capsids and the effects of capsid protein mutations on elasticity and strength. Proc. Natl Acad. Sci. USA 103, 6184–6189 (2006).
Roos, W. H. AFM nanoindentation of protein shells, expanding the approach beyond viruses. Semin. Cell Dev. Biol. 73, 145–152 (2018).
Peng, K., Qin, F. G., Jiang, R., Qu, W. & Wang, Q. Production and dispersion of free radicals from transient cavitation bubbles: an integrated numerical scheme and applications. Ultrason. Sonochem. 88, 106067. https://doi.org/10.1016/j.ultsonch.2022.106067 (2022).
Sadraeian, M., Kabakova, I., Zhou, J. & Jin, D. Virus inactivation by matching the vibrational resonance. Appl. Phys. Rev. 11, 21324. https://doi.org/10.1063/5.0183276/3295468 (2024).
Davis, P. J. & Williams, S. C. Protein modification by thermal processing. Allergy 53, 102–105. https://doi.org/10.1111/j.1398-9995.1998.tb04975.x (1998).
Slavikova, M. et al. Heat inactivation by high temperature as an approach to combat airborne pathogens. Acta Virol. 67, 11640. https://doi.org/10.3389/av.2023.11640 (2023).
Soto-Torres, E. et al. Ultrasound and Doppler findings in pregnant women with SARS-CoV-2 infection. Ultrasound Obstet. Gynecol. 58, 111. https://doi.org/10.1002/uog.23642 (2021).
Kim, J. H. et al. Possible effects of radiofrequency electromagnetic field exposure on central nervous system. Biomol. Ther. 27, 265. https://doi.org/10.4062/biomolther.2018.152 (2018).
O’Brien, W. D. Ultrasound–biophysics mechanisms. Prog. Biophys. Mol. Biol. 93, 212–255. https://doi.org/10.1016/j.pbiomolbio.2006.07.010 (2007).
Stetefeld, J., McKenna, S. A. & Patel, T. R. Dynamic light scattering: a practical guide and applications in biomedical sciences. Biophys. Rev. 8, 409. https://doi.org/10.1007/s12551-016-0218-6 (2016).
Caputo, F. et al. Measuring particle size distribution of nanoparticle-enabled medicinal products: the joint view of EUNCL and NCI-NCL. J. Control. Release 299, 31–43. https://doi.org/10.1016/j.jconrel.2019.02.030 (2019).
Acknowledgements
O.M.B thanks Daniela Gravina Stamato for discussions on clinical ultrasonography and early suggestions.
Funding
FAPESP: F.P.V 2020/07645–0; G.N 2023/07241–5; F.Q.C 2013/08216–2 and 2020/05601–6; E.A 2019/26119–0; O.M.B 2018/22214–6, 2021/08325–2, and 2023/07241–5. O.M.B also acknowledges CNPq 05610/2022–8. F.P.V also acknowledges and Institut Merrieux Grant.
Author information
Authors and Affiliations
Contributions
F.P.V, G.N, and O.M.B designed the study. F.P.V performed immunofluorescence and confocal analyses. R.M performed TCID50 assays. F.P.V and G.N performed ultrasound exposures. G.N and M.A.P.S performed SEM and AFM analyses. G.C.M.R and C.J.L.C performed, analyzed, and discussed DLS experiments. F.Q.C, E.A, and O.M.B provided critical materials and comments. F.P.V, G.N, and O.M.B wrote the manuscript. O.M.B coordinated and supervised the project. All authors approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Veras, F.P., Nakamura, G., Pereira-da-Silva, M.A. et al. Ultrasound effectively destabilizes and disrupts the structural integrity of enveloped respiratory viruses. Sci Rep (2026). https://doi.org/10.1038/s41598-026-37584-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-37584-x