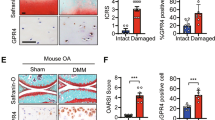
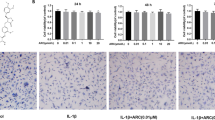
Abstract
Osteoarthritis (OA) is a prevalent joint degenerative disease involving inflammation and oxidative stress, with reactive oxygen species (ROS) driving progression. Restoring joint redox balance mitigates cartilage damage. Osilyhizidine (OSR), from Sophora alopecuroides, has anti-inflammatory/antioxidant properties, but its OA-specific effects and mechanisms were unclear. In vitro experiments assessed OSR’s impact on OA chondrocyte proliferation, repair, and inflammation, focusing on Glutathione peroxidase 4 (GPX4) and solute carrier family 7 member 11 (SLC7A11) regulation. A murine OA model validated findings in vivo. OSR showed anti-inflammatory, antioxidant effects and promoted cartilage repair, enhancing chondrocyte functions under inflammation and suppressing pro-inflammation. It upregulated GPX4 (improving ROS detoxification) and SLC7A11 (facilitating glutathione synthesis for redox balance) at transcriptional and protein levels. These were confirmed in mice. OSR alleviates OA by activating GPX4/SLC7A11 to regulate ROS and oxidative stress, emerging as a promising OA therapeutic candidate, offering insights into redox-targeted interventions.
Similar content being viewed by others
Data availability
All data generated or analyzed during this study are available from the corresponding author upon reasonable request. The datasets include raw Western blot images, RT-qPCR Ct values, flow cytometry files, histological and immunohistochemical images, and molecular docking results. All data have been carefully validated, and the authors affirm their accuracy and reliability. Requests for access should be directed to the corresponding author.
Abbreviations
- CETSA:
-
Cellular thermal shift assay
- COX‑2:
-
Cyclooxygenase‑2
- DMSO:
-
Dimethyl sulfoxide
- GPX4:
-
Glutathione peroxidase 4
- GSH:
-
Glutathione
- H&E:
-
Hematoxylin and eosin
- IL‑6:
-
Interleukin‑6
- MDA:
-
Malondialdehyde
- MMP‑13:
-
Matrix metallopeptidase 13
- OA:
-
Osteoarthritis
- OSR:
-
Oxysophoridine
- ROS:
-
Reactive oxygen species
- SLC7A11:
-
Solute carrier family 7 member 11
- TNF‑α:
-
Tumor necrosis factor‑α
- 4‑HNE:
-
4‑Hydroxynonenal
References
Singh, N., Bhattacharjee, A., Kumar, P. & Katti, D. S. Targeting multiple disease hallmarks using a synergistic disease-modifying drug combination ameliorates osteoarthritis via inhibition of senescence and inflammation. Life Sci. 334, 122212 (2023).
Yu, H. et al. Supramolecular self-assembly of egcg-selenomethionine nanodrug for treating osteoarthritis. Bioact. Mater. 32, 164–176 (2024).
Sahu, N. et al. Multi-parametric profiling of circulating immune cells identifies an expansion of cd25(hi) switched memory b cells in osteoarthritis. Arthritis Rheumatol. (2025).
Dai, Z. et al. Gamma-oryzanol alleviates osteoarthritis development by targeting keap1–nrf2 binding to interfere with chondrocyte ferroptosis. Int. Immunopharmacol. 128, 111469 (2024).
Gao, Q. et al. Associated factors of sarcopenia in community-dwelling older adults: a systematic review and meta-analysis. Nutrients 13(12) (2021).
Ruan, Q., Wang, C., Zhang, Y. & Sun, J. Brevilin a attenuates cartilage destruction in osteoarthritis mouse model by inhibiting inflammation and ferroptosis via sirt1/nrf2/gpx4 signaling pathway. Int. Immunopharmacol. 124, 110924 (2023).
He, L. et al. Gprc5b protects osteoarthritis by regulation of autophagy signaling. Acta Pharm. Sin. B. 13(7), 2976–2989 (2023).
Lin, S. et al. Plantamajoside suppresses the activation of nf-κb and mapk and ameliorates the development of osteoarthritis. Int. Immunopharmacol. 115, 109582 (2023).
Qiu, J. et al. Neratinib exerts dual effects on cartilage degradation and osteoclast production in osteoarthritis by inhibiting the activation of the mapk/nf-κb signaling pathways. Biochem. Pharmacol. 205, 115155 (2022).
Shi, Y. et al. Tangeretin suppresses osteoarthritis progression via the nrf2/nf-κb and mapk/nf-κb signaling pathways. Phytomedicine Stuttgart 98, 153928 (2022).
Xu, Y. et al. Atractylenolide-iii alleviates osteoarthritis and chondrocyte senescence by targeting nf-κb signaling. Phytother. Res. 37(10), 4607–4620 (2023).
Gan, X. et al. Vaccarin ameliorates osteoarthritis by suppressing the c-jun n-terminal kinase (jnk)-serum amyloid a2 (saa2) pathway mediating chondrocyte senescence. Phytomedicine 141, 156697 (2025).
Gou, Y. et al. Targeted activation on bnip3 enhances mitophagy to prevent the progression of osteoarthritis. J. Orthop. Transl. 51, 242–255 (2025).
Chen, J. Y. et al. Oxysophoridine inhibits oxidative stress and inflammation in hepatic fibrosis via regulating nrf2 and nf-κb pathways. Phytomedicine 132, 155585 (2024).
Wang, H. et al. Protective effect of oxysophoridine on cerebral ischemia/reperfusion injury in mice. Neural Regen. Res. 8(15), 1349–1359 (2013).
Wang, Y. S. et al. Anti-inflammation effects of oxysophoridine on cerebral ischemia-reperfusion injury in mice. Inflammation 38(6), 2259–2268 (2015).
Liu, Y. et al. Pantothenic acid alleviates osteoarthritis progression by inhibiting inflammatory response and ferroptosis through the sirt1/nrf2 signaling pathway. Chem. Biol. Interact. 413, 111494 (2025).
Lan, W. et al. Ugdh lactylation aggravates osteoarthritis by suppressing glycosaminoglycan synthesis and orchestrating nucleocytoplasmic transport to activate mapk signaling. Adv. Sci., e2413709 (2025).
Xie, Y. et al. Jp4-039 protects chondrocytes from ferroptosis to attenuate osteoarthritis progression by promoting pink1/parkin-dependent mitophagy. J. Orthop. Transl. 51, 132–144 (2025).
Chen, Y. et al. Ros fueled autonomous sol-gel-sol transitions for on-demand modulation of inflammation in osteoarthritis. J. Control. Release 379, 1006–1021 (2025).
Lee, S. H., Shin, M. K. & Sung, J. S. Tamarixetin protects chondrocytes against il-1β-induced osteoarthritis phenotype by inhibiting nf-κb and activating nrf2 signaling. Antioxidants 13(10) (2024).
Xiang, J. et al. Nir-enhanced pt single atom/g-c(3)n(4) nanozymes as sod/cat mimics to rescue atp energy crisis by regulating oxidative phosphorylation pathway for delaying osteoarthritis progression. Bioact. Mater. 36, 1–13 (2024).
Xu, X. X. et al. Theaflavin protects chondrocytes against apoptosis and senescence via regulating nrf2 and ameliorates murine osteoarthritis. Food Funct. 12(4), 1590–1602 (2021).
Chen, Y. R. et al. The chondroprotective effect of diosmin on human articular chondrocytes under oxidative stress. Phytother. Res. 33(9), 2378–2386 (2019).
Zhou, X. et al. Antioxidant taurine inhibits chondrocyte ferroptosis through upregulation of ogt/gpx4 signaling in osteoarthritis induced by anterior cruciate ligament transection. J. Adv. Res. (2025).
Wang, J. et al. Vinpocetine protects against osteoarthritis by inhibiting ferroptosis and extracellular matrix degradation via activation of the nrf2/gpx4 pathway. Phytomedicine 135, 156115 (2024).
Song, C. et al. Molecular mechanisms of immunoinflammatory infiltration and ferroptosis in arthritis revealed by a combination of bioinformatics and single-cell analysis. J. Inflamm. Res. 18, 2409–2432 (2025).
Zhang, M. et al. Synchronously evoking disulfidptosis and ferroptosis via systematical glucose deprivation targeting slc7a11/gsh/gpx4 antioxidant axis. ACS Nano 19(14), 14233–14248 (2025).
Zhao, L. et al. Lactate dehydrogenase b noncanonically promotes ferroptosis defense in kras-driven lung cancer. Cell Death Differ. 32(4), 632–645 (2025).
Ahn, J. et al. 4–1bb stimulation with concomitant inactivation of adenosine a2b receptors enhances cd8+ t cell antitumor response. J. Clin. Invest. (2025).
Ma, X. et al. Apatinib combined with paclitaxel suppresses synergistically tnbc progression through enhancing ferroptosis susceptibility regulated slc7a11/gpx4/acsl4 axis. Cell. Signal. 131, 111760 (2025).
Li, X. et al. Oxymatrine inhibits melanoma development by modulating the immune microenvironment and targeting the myc/pd-l1 pathway. Int. Immunopharmacol. 124(Pt B), 111000 (2023).
Li, N. et al. Vasorelaxation effect of oxysophoridine on isolated thoracicc aorta rings of rats. Chin. J. Physiol. 64(6), 274–280 (2021).
DiMartino, S. J. et al. Efficacy and safety of fasinumab in an nsaid-controlled study in patients with pain due to osteoarthritis of the knee or hip. BMC Musculoskelet. Disord. 26(1), 192 (2025).
Hameed, F. & Ihm, J. Injectable medications for osteoarthritis. PM&R 4(5 Suppl), S75–S81 (2012).
Wang, H. et al. Ro5126766 attenuates osteoarthritis by inhibiting osteoclastogenesis and protecting chondrocytes through mediating the erk pathway. J. Orthop. Transl. 52, 27–39 (2025).
Binvignat, M. & Sellam, J. Molecular endotypes and theratypes in osteoarthritis: transforming a concept into reality with deep learning and multiomics. Ann. Rheum. Dis. (2025).
Jin, S. J. et al. In vivo and in vitro induction of the apoptotic effects of oxysophoridine on colorectal cancer cells via the bcl-2/bax/caspase-3 signaling pathway. Oncol. Lett. 14(6), 8000–8006 (2017).
Rui, C. et al. Anti-apoptotic and neuroprotective effects of oxysophoridine on cerebral ischemia both in vivo and in vitro. Planta Med. 79(11), 916–923 (2013).
Wang, T. et al. Effects of oxysophoridine on amino acids after cerebral ischemic injury in mice. Ann. Indian Acad. Neurol. 17(3), 313–316 (2014).
Acknowledgements
The authors gratefully acknowledge financial support from the Health Research Program of Anhui Province (Grant No. AHWJ2024Aa20135).
Funding
This work was supported by the Health Research Program of Anhui (Grant No. AHWJ2024Aa20135).
Author information
Authors and Affiliations
Contributions
Jun Tu (First Author): Conceptualization, Methodology, Software, Investigation, Formal Analysis, Writing—Original Draft, Writing—Review & Editing; Zhiwei Peng: Data Curation, Writing—Original Draft, Writing—Review & Editing; Haobo Liu: Visualization, Investigation, Writing—Review & Editing; Xiyang Sun :Visualization, Writing—Review & Editing; Jianqi Zhao: Resources, Supervision, Writing—Review & Editing; Bin Xu: Software, Validation, Writing—Review & Editing; XuZhu: Concepualization,Methodology, Writing—review Editing. Baofang Liu:(Corresponding Author): Conceptualization, Funding Acquisition, Resources, Supervision, Writing—Review Editing. All authors agreed on the journal for submission, reviewed and approved all versions of the manuscript, and accept responsibility for the published content.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent for publication
Written informed consent for publication was obtained from all participants who provided human cartilage samples. All identifying information has been removed to ensure anonymity, and signed consent forms are available for review by the journal’s editorial office upon request.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tu, J., Peng, Z., Sun, X. et al. Oxysophoridine promotes osteoarthritis repair via GSH system activation and ROS suppression. Sci Rep (2026). https://doi.org/10.1038/s41598-026-37912-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-37912-1