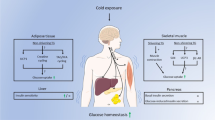
Abstract
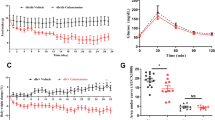
Acute metabolic acidosis (MA), a feature mostly associated with chronic kidney disease, decreases glucose tolerance and insulin sensitivity. By contrast, the effects of chronic MA on glucose homeostasis remain elusive. Here, we evaluated glucose homeostasis and metabolic parameters in male mice exhibiting chronic MA via long-term NH4Cl administration. Unlike acute MA, chronic MA resulted in lower body weight, increased energy expenditure, lower basal glycemia, improved glucose tolerance without changes in insulin secretion or sensitivity. No overall glucose uptake changes were observed. However, hepatic and intestinal gluconeogenesis were decreased whereas renal endogenous glucose production was increased in mice with chronic MA. The elevated glucose urinary excretion was associated with lower expression of renal sodium/glucose co-transporters. Transcriptomic analysis revealed that chronic MA potentiates anion transport, glucose and lipid metabolism, mitochondrial and oxidative phosphorylation pathways in the kidney. Overall, chronic MA improves glucose tolerance without changes in insulin secretion or sensitivity, likely due to reduced hepatic gluconeogenesis, decreased renal glucose reabsorption and increased energy demands in the kidney.
Similar content being viewed by others
Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its Supplementary material. Raw data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
Jager, K. J. et al. A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Nephrol. Dial. Transplant 34 (11), 1803–1805 (2019).
Burger, M. Metabolic Acidosis (StatPearls Publishing, 2023).
Chan, J. Y. M., Islahudin, F., Tahir, N. A. M., Makmor-Bakry, M. & Tan, C. H. H. Prevalence, risk factors, and management of metabolic acidosis in chronic kidney disease patients: A multicenter retrospective study in Malaysia. Cureus 16 (3), e56314 (2024).
Adamczak, M. et al. Diagnosis and treatment of metabolic acidosis in patients with chronic kidney disease - position statement of the working group of the polish society of nephrology. Kidney Blood Press Res. 43, 959–962 (2018).
Raphael, K. L. Metabolic acidosis in CKD: core curriculum. Am. J. Kidney Dis. 74, 263–275 (2019).
Souto, G., Donapetry, C., Calvino, J. & Adeva, M. M. Metabolic acidosis-induced insulin resistance and cardiovascular risk. Metab. Syndr. Relat. Disord. 9, 247–253 (2011).
Bellasi, A. et al. Correction of metabolic acidosis improves insulin resistance in chronic kidney disease. BMC Nephrol. 17 (1), 158 (2016).
DiNicolantonio, J. J. & O’Keefe, J. H. Low-grade metabolic acidosis as a driver of insulin resistance. Open Heart 8 (2), (2021).
Kim, H. J. Metabolic Acidosis in chronic kidney disease: pathogenesis, clinical consequences, and treatment. Electrolyte Blood Press 19 (2), 29–37 (2021).
Kriz, W. & LeHir, M. Pathways to nephron loss starting from glomerular diseases-insights from animal models. Kidney Int. 67 (2), 404–419 (2005).
Weiner, I. D. & Verlander, J. W. Renal ammonia metabolism and transport. Compr. Physiol. 3 (1), 201–220 (2013).
Kraut, J. A. & Madias, N. E. Metabolic acidosis: pathophysiology, diagnosis and management. Nat. Rev. Nephrol. 6 (5), 274–285 (2010).
Li, S. et al. Are low levels of serum bicarbonate associated with risk of progressing to impaired fasting glucose/diabetes? A single-centre prospective cohort study in Beijing, China. BMJ Open 8 (7), e019145 (2018).
Mackler, B., Lichenstein, H. & Guest, G. M. Effects of ammonium chloride acidosis on glucose tolerance in dogs. Am. J. Physiol. 168 (1), 125–130 (1952).
Guest, G., Mackler, B. & Knowles, H. J. Effects of acidosis on insulin action and on carbohydrate and mineral metabolism. Diabetes 1 (4), 276–282 (1952).
Weisinger, J., Swenson, R., Greene, W., Taylor, J. & Reaven, G. Comparison of the effects of metabolic acidosis and acute uremia on carbohydrate tolerance. Diabetes 21 (11), 1109–1115 (1972).
Haldane, J. B. S., Wigglesworth, V. B. & Woodrow, C. The effect of reaction changes on human carbohydrate and oxygen metabolism. Proc. R. Soc. London Ser. B, Containing Papers Biol. Charact. 96 (672), 15–28 (1924).
DeFronzo, R. & Beckles, A. Glucose intolerance following chronic metabolic acidosis in man. Am. J. Physiol. 236 (4), 328–334 (1979).
Franch, H. A. et al. Acidosis impairs insulin receptor substrate-1-associated phosphoinositide 3-kinase signaling in muscle cells: consequences on proteolysis. Am. J. Physiol. Renal Physiol. 287 (4), F700–F706 (2004).
May, R., Masud, T., Logue, B., Bailey, J. & England, B. Chronic metabolic acidosis accelerates whole body proteolysis and oxidation in awake rats. Kidney Int. 41 (6), 1535–1542 (1992).
Whittaker, J., Cuthbert, C., Hammond, V. & Alberti, K. The effects of metabolic acidosis in vivo on insulin binding to isolated rat adipocytes. Metabolism 31 (6), 553–557 (1982).
Teta, D. et al. Acidosis downregulates leptin production from cultured adipocytes through a glucose transport-dependent post-transcriptional mechanism. J. Am. Soc. Nephrol. 14 (9), 2248–2254 (2003).
Disthabanchong, S. et al. Metabolic acidosis lowers circulating adiponectin through inhibition of adiponectin gene transcription. Nephrol. Dial Transplant. 26 (2), 592–598 (2011).
Igarashi, M. et al. Effect of acidosis on insulin binding and glucose uptake in isolated rat adipocytes,". Tohoku J. Exp. Med. 169 (3), 205–213 (1993).
Mutel, E. et al. Control of blood glucose in the absence of hepatic glucose production during prolonged fasting in mice: induction of renal and intestinal gluconeogenesis by glucagon. Diabetes 60 (12), 3121–3131 (2011).
Ferrannini, E. Learning from glycosuria. Diabetes 60 (3), 695–696 (2011).
Johnsen, L. Ø., Sigad, A., Friis, K. A., Berg, P. M. & Damkier, H. H. NH4Cl-induced metabolic acidosis increases the abundance of HCO3- transporters in the choroid plexus of mice. Front Physiol 15, 1491793 (2024).
Nowik, M., Kampik, N. B., Mihailova, M., Eladari, D. & Wagner, C. A. Induction of metabolic acidosis with ammonium chloride (NH4Cl) in mice and rats–species differences and technical considerations. Cell Physiol. Biochem. 26 (6), 1059–1072 (2010).
Fang, Y. W. et al. Chronic metabolic acidosis activates renal tubular sodium chloride cotransporter through angiotension II-dependent WNK4-SPAK phosphorylation pathway. Sci. Rep. 6, 18360 (2016).
Mannon, E. C. et al. Renal mass reduction increases the response to exogenous insulin independent of acid-base status or plasma insulin levels in rats. Am. J. Renal Physiol. 321 (4), F494–F504 (2021).
Jakab, M. et al. The H+/K+ ATPase inhibitor SCH-28080 Inhibits insulin secretion and induces cell death in INS-1E rat insulinoma cells. Cell Physiol. Biochem. 43 (3), 1037–1051 (2017).
Imenez Silva, P. H. & Mohebbi, N. Kidney metabolism and acid-base control: back to the basics. Pflugers Arch. 474 (8), 919–934 (2022).
Iles, R., Cohen, R., Rist, A. & Baron, P. The mechanism of inhibition by acidosis of gluconeogenesis from lactate in rat liver. Biochem. J. 164 (1), 185–191 (1977).
Beech, J., Iles, R. & Cohen, R. Bicarbonate in the treatment of metabolic acidosis: effects on hepatic intracellular pH, gluconeogenesis, and lactate disposal in rats. Metabolism 42 (3), 341–346 (1993).
Minarik, P., Tomaskova, N., Kollarova, M. & Antalik, M. Malate dehydrogenases–structure and function. Gen. Physiol. Biophys. 21 (3), 257–265 (2002).
Aamer, E. et al. Influence of electrode potential, pH and NAD(+) concentration on the electrochemical NADH regeneration. Sci. Rep. 12 (1), 16380 (2022).
She, P. et al. Mechanisms by which liver-specific PEPCK knockout mice preserve euglycemia during starvation. Diabetes 52 (7), 1649–1654 (2003).
Burgess, S. et al. Impaired tricarboxylic acid cycle activity in mouse livers lacking cytosolic phosphoenolpyruvate carboxykinase. J. Biol. Chem. 279 (47), 48941–48949 (2004).
Baldini, N. & Avnet, S. The effects of systemic and local acidosis on insulin resistance and signaling. Int. J. Mol. Sci. 20 (1), 126 (2019).
Holeček, M. Origin and roles of alanine and glutamine in gluconeogenesis in the liver, kidneys, and small intestine under physiological and pathological conditions. Int. J. Mol. Sci. 25 (13), 7037 (2024).
Mithieux, G., Rajas, F. & Gautier-Stein, A. A novel role for glucose 6-phosphatase in the small intestine in the control of glucose homeostasis. J. Biol. Chem. 279 (43), 44231–44234 (2004).
Fu, J., Liu, S. & Li, M. Optimal fasting duration for mice as assessed by metabolic status. Sci. Rep. 14, 21509 (2024).
Carper, D., Coué, M., Laurens, C., Langin, D. & Moro, C. Reappraisal of the optimal fasting time for insulin tolerance tests in mice. Mol. Metab. 42, 101058 (2020).
Lee, Y., Lee, Y. & Han, H. Regulatory mechanisms of Na(+)/glucose cotransporters in renal proximal tubule cells. Kidney Int. Suppl. 72 (106), S27–S35 (2007).
Liman, M. N. P. & Jialal, I. Physiology, Glycosuria (StatPearls Publishing, 2023).
Zhang, J. et al. Macula densa SGLT1-NOS1-tubuloglomerular feedback pathway, a new mechanism for glomerular hyperfiltration during hyperglycemia. J. Am. Soc. Nephrol. 30 (4), 578–593 (2019).
Caudron-Herger, M. & Diederichs, S. 2021 Insights from the degradation mechanism of cyclin D into targeted therapy of the cancer cell cycle. Signal Transduct. Target Ther. 6 (1), 311 (2021).
Guo, Z. Y. et al. The elements of human cyclin D1 promoter and regulation involved. Clin. Epigenet. 2 (2), 63–76 (2011).
Pontoglio, M. et al. HNF1alpha controls renal glucose reabsorption in mouse and man. EMBO Rep. 1 (4), 359–365 (2000).
Freitas, H. et al. Na(+) -glucose transporter-2 messenger ribonucleic acid expression in kidney of diabetic rats correlates with glycemic levels: involvement of hepatocyte nuclear factor-1alpha expression and activity. Endocrinology 149 (2), 717–724 (2008).
Takesue, H., Hirota, T., Tachimura, M., Tokashiki, A. & Ieiri, I. Nucleosome positioning and gene regulation of the SGLT2 gene in the renal proximal tubular epithelial cells. Mol. Pharmacol. 94 (3), 953–962 (2018).
Bonner, C. et al. Inhibition of the glucose transporter SGLT2 with dapagliflozin in pancreatic alpha cells triggers glucagon secretion. Nat. Med. 21 (5), 512–517 (2015).
Marable, S. S., Chung, E. & Park, J.-S. Hnf4a is required for the development of Cdh6-expressing progenitors into proximal tubules in the mouse kidney. J. Am. Soc. Nephrol. 31 (11), 2543–2558 (2020).
Fu, M. et al. Epoxyeicosatrienoic acids improve glucose homeostasis by preventing NF-kappaB-mediated transcription of SGLT2 in renal tubular epithelial cells. Mol. Cell Endocrinol. 523, 111149 (2021).
Tang, L. et al. Dapagliflozin slows the progression of the renal and liver fibrosis associated with type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 313 (5), E563–E576 (2017).
Terami, N. et al. Long-term treatment with the sodium glucose cotransporter 2 inhibitor, dapagliflozin, ameliorates glucose homeostasis and diabetic nephropathy in db/db mice. PLoS ONE 9 (6), e100777 (2014).
Yao, D., Wang, S., Wang, M. & Lu, W. Renoprotection of dapagliflozin in human renal proximal tubular cells via the inhibition of the high mobility group box 1-receptor for advanced glycation end products-nuclear factor-kappaB signaling pathway. Mol. Med. Rep. 18 (4), 3625–3630 (2018).
Faivre, A., Verissimo, T., Auwerx, H., Legouis, D. & de Seigneux, S. Tubular cell glucose metabolism shift during acute and chronic injuries. Front Med (Lausanne) 8, 742072 (2021).
Gao, Y., Nelson, D. W., Banh, T., Yen, M. I. & Yen, C. L. E. Intestine-specific expression of MOGAT2 partially restores metabolic efficiency in Mogat2-deficient mice. J. Lipid Res. 54 (6), 1644–1652 (2013).
Lin, P. H. & Duann, P. Dyslipidemia in kidney disorders: perspectives on mitochondria homeostasis and therapeutic opportunities. Front. Physiol. 11, 1050 (2020).
Sanz, A. B. et al. NF-kappaB in renal inflammation. J. Am. Soc. Nephrol. 21 (8), 1254–1262 (2010).
Verissimo, T. et al. PCK1 is a key regulator of metabolic and mitochondrial functions in renal tubular cells. Am. J. Physiol. Renal Physiol. 324 (6), F532–F543 (2023).
Wang, G. et al. Fat storage-inducing transmembrane proteins: beyond mediating lipid droplet formation. Cell Mol. Biol. Lett. 27 (1), 98 (2022).
Bhargava, P. & Schnellmann, R. G. Mitochondrial energetics in the kidney. Nat. Rev. Nephrol. 13 (10), 629–646 (2017).
Gumz, M. L., Lynch, I. J., Greenlee, M. M., Cain, B. D. & Wingo, C. S. The renal H+-K+-ATPases: physiology, regulation, and structure. Am. J. Physiol. Renal Physiol. 298 (1), F12-21 (2010).
Zhao, L. et al. Energy metabolic reprogramming regulates programmed cell death of renal tubular epithelial cells and might serve as a new therapeutic target for acute kidney injury. Front. Cell Dev. Biol. 11, 1276217 (2023).
Greenbaum, D., Colangelo, C., Williams, K. & Gerstein, M. Comparing protein abundance and mRNA expression levels on a genomic scale. Genome Biol. 4 (9), 117 (2003).
Nowik, M. et al. Genome-wide gene expression profiling reveals renal genes regulated during metabolic acidosis. Physiol. Genom. 32 (3), 322–334 (2008).
Huynh, N. V., Rehage, C. & Hyndman, K. A. Mild dehydration effects on the murine kidney single-nucleus transcriptome and chromatin accessibility. Am. J. Physiol. Renal Physiol. 325 (6), F717–F732 (2023).
Annicotte, J. S. et al. The CDK4-pRB-E2F1 pathway controls insulin secretion. Nat. Cell Biol. 11 (8), 1017–1023 (2009).
Lopez-Cayuqueo, K. I. et al. A mouse model of pseudohypoaldosteronism type II reveals a novel mechanism of renal tubular acidosis. Kidney Int. 94 (3), 514–523 (2018).
Acknowledgements
The authors thank the Experimental Resources platform from Lille University, especially CDegraeve, JVergoten, RDehaynin and JDevassine for animal care. We also thank LPinot, and SMeulebrouck for helpful discussions and technical assistance. We would also like to thank Dr Gilles Mithieux and Dr Fabienne Rajas for the anti-G6PC antibody kindly gifted under the Open Material Transfer Agreement (MTA) regulations.
Funding
This work was supported by grants from the French National Research Agency: ANR-16-CE14-0031 to RC, from the Association pour l’utilisation du rein artificiel à la Réunion (AURAR)-Philancia to RC, from the Soutien à l'accueil de Talents de la Recherche Scientifique (STaRS), Région Hauts-de-France to RC, the French National Research Agency (ANR-10-LABX-46 [European Genomics Institute for Diabetes] to PF and AB and ANR-10-EQPX-07-01 [LIGAN-PM]) to PF and AB, and from the National Center for Precision Diabetic Medicine – PreciDIAB, which is jointly supported by the French National Agency for Research (ANR-18-IBHU-0001) to PF and AB, by the European Union (FEDER), by the Hauts-de-France Regional Council and by the European Metropolis of Lille (MEL). AB holds an ERC consolidator grant (OpiO, contract number 101043671).
Author information
Authors and Affiliations
Contributions
NZ, JB-V and RC conceptualized the study. NZ, JMontaigne, JMerrheim, CD, EC, FA, JB-V, ED, BT and RC performed the investigations. NZ, SA and MD did the formal analyses and data curation. EC, BS, PF, AB and RC contributed to resources. NZ, AB and RC contributed to data visualization. NZ, CB and AB wrote the first draft. NZ and CB supervised the conception of the article. All authors revised the manuscript for intellectual content, and read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zaibi, N., Montaigne, J., Baraka-Vidot, J. et al. Chronic NH4Cl loading improves glucose tolerance without modifying insulin sensitivity in mice. Sci Rep (2026). https://doi.org/10.1038/s41598-026-38007-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-38007-7