Abstract
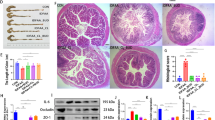
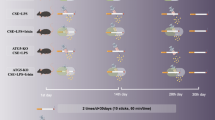
Shenling Baizhu Powder (SLBZP) is a prominent formulation widely used in the treatment of pulmonary diseases. However, studies examining the mechanisms of SLBZP for treating asthma are limited. This study aimed to clarify the efficacy and possible mechanisms of SLBZP in the context of asthma from the perspective of gut microbiota-metabolism-immune crosstalk. Key parameters including airway hyperresponsiveness, lung pathological features and the expression of inflammatory mediators from Th2 and Th17 cells were employed to validate the anti-inflammatory properties of SLBZP. The anti-asthma mechanism of SLBZP was investigated using metagenomic sequencing, metabolomics, flow cytometry, RT-qPCR, immunohistochemistry (IHC) and immunofluorescence (IF). SLBZP demonstrated significant capacity to mitigate histopathological alterations associated with ovalbumin-induced asthma and suppress the secretion of inflammatory mediators (IL-4, IL-5, IL-13 and IL-17A) in BALF. Metagenomic results demonstrated that the protective effects of SLBZP were primarily associated with Ligilactobacillus, Eubacterium and Clostridium. Additionally, metabolomics results identified that three vital metabolic pathways were substantially regulated by SLBZP in asthmatic mice, especially D-glutamine and -glutamate metabolism. Furthermore, IHC and IF results showed that SLBZP significantly inhibited the expression of GLS1 and GOT1, which inhibited the conversion of L-glutamine to α-ketoglutarate and regulated the imbalance of Th1/Th2 and Treg/Th17. RT-qPCR results showed that SLBZP promoted the expressions of T-bet, IFN-γ, IL-10 and Foxp3 mRNA, and inhibited the expression of GATA3, IL-4, IL-5, IL-13, IL-17A and RORγt mRNA. The findings from flow cytometry provided additional evidence. Thus, this modulated the imbalance of Th1/Th2 and Treg/Th17 and exerted the immunomodulatory properties of SLBZP. SLBZP exerted protective effects against OVA-induced asthma and modified the structure and functional characteristics of the gut microbiota, and serum metabolite profiles in asthmatic mice. The anti-asthma mechanism of SLBZP may be associated with the modulation of the gut microbiota and Glutamine-GLS1 pathway.
Similar content being viewed by others
Data availability
The main data generated or analyzed during this study are included in this manuscript and its Additional files and are available from the corresponding author on reasonable request. Metagenomic data generated during the current study have been deposited in NCBI (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA1227753).
Abbreviations
- OVA:
-
Ovalbumin
- SLBZP:
-
Shenling Baizhu Powder
- TCM:
-
Traditional Chinese medicine
- BALF:
-
Bronchoalveolar lavage fluid
- AHR:
-
Airway hyperresponsiveness
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- ROC:
-
Receiver operating characteristic
- AUC:
-
Area under curve
References
Huang, K. et al. Prevalence, risk factors, and management of asthma in China: A national cross-sectional study. Lancet 394, 407–418. https://doi.org/10.1016/s0140-6736(19)31147-x (2019).
Reddel, H. K. et al. Global initiative for asthma strategy 2021: Executive summary and rationale for key changes. Am. J. Respir. Crit. Care Med. 205, 17–35. https://doi.org/10.1164/rccm.202109-2205PP (2022).
Valero, A. L., Trigueros, J. A. & Plaza, V. Multidisciplinary consensus on inhaled therapy in asthma. Expert Rev. Respir. Med. 15, 425–434. https://doi.org/10.1080/17476348.2021.1841639 (2021).
Oray, M., Abu Samra, K., Ebrahimiadib, N., Meese, H. & Foster, C. S. Long-term side effects of glucocorticoids. Expert Opin. Drug Saf. 15, 457–465. https://doi.org/10.1517/14740338.2016.1140743 (2016).
Wang, H. Clinical study on the improvement of stable chronic obstructive pulmonary disease with Shenling Baizhu powder. Yunnan J. Tradit. Chin. Med. Materia Medica 31, 14–16. https://doi.org/10.16254/j.cnki.53-1120/r.2010.09.045 (2010).
Liu, Z. & Chen, Y. Effect analysis of Shenlingbaizhu powder on stable COPD. Guangming J. Chin. Med. 32, 832–834 (2017).
Lei, Y. Effect of Shenling Baizhu powder in the treatment of remission stage of asthma. World Latest Med. Inf. 19, 171. https://doi.org/10.19613/j.cnki.1671-3141.2019.80.081 (2019).
Jin, S. & Zhang, H. Clinical efficacy of Shenling Baizhu powder in the treatment of remission stage of asthma of lung spleen qi deficiency type in children. Henan Med. Res. 29, 3961–3963 (2020).
Wang, C. et al. Research progress of Shenling Baizhu powder in the treatment of respiratory diseases. Clin. J. Tradit. Chin. Med. 32, 1178–1182. https://doi.org/10.16448/j.cjtcm.2020.0649 (2020).
Ouyang, X. & Xu, H. Inhibitory effect of Shen ling Baizhu powder on airway inflammation in young asthmatic rats. Tradit. Chin. Drug Res. Clin. Pharmacol. 30, 812–815. https://doi.org/10.19378/j.issn.1003-9783.2019.07.010 (2019).
Ouyang, X. & Xu, H. in The 23rd National Pediatric Integrated Traditional Chinese and Western Medicine Academic Conference (2019).
Li, Y. et al. Shenling Baizhu Decoction treats ulcerative colitis of spleen-deficiency and dampness obstruction types by targeting ‘gut microbiota and galactose metabolism-bone marrow’ axis. J. Ethnopharmacol. 335, 118599. https://doi.org/10.1016/j.jep.2024.118599 (2024).
Chen, D. et al. Shenling Baizhu San ameliorates non-alcoholic fatty liver disease in mice by modulating gut microbiota and metabolites. Front. Pharmacol. 15, 1343755. https://doi.org/10.3389/fphar.2024.1343755 (2024).
Wang, J. et al. Forsythiaside A alleviates acute lung injury by inhibiting inflammation and epithelial barrier damages in lung and colon through PPAR-γ/RXR-α complex. J. Adv. Res. 60, 183–200. https://doi.org/10.1016/j.jare.2023.08.006 (2024).
Du, C., Zhao, Y., Shen, F. & Qian, H. Effect of brassica rapa L. polysaccharide on lewis lung cancer mice by inflammatory regulation and gut microbiota modulation. Foods https://doi.org/10.3390/foods13223704 (2024).
Paone, P. et al. Prebiotic oligofructose protects against high-fat diet-induced obesity by changing the gut microbiota, intestinal mucus production, glycosylation and secretion. Gut Microbes 14, 2152307. https://doi.org/10.1080/19490976.2022.2152307 (2022).
Feng, X. et al. Glutaminolysis and CD4(+) T-cell metabolism in autoimmunity: From pathogenesis to therapy prospects. Front. Immunol. 13, 986847. https://doi.org/10.3389/fimmu.2022.986847 (2022).
Kono, M., Yoshida, N., Maeda, K. & Tsokos, G. C. Transcriptional factor ICER promotes glutaminolysis and the generation of Th17 cells. Proc. Natl. Acad. Sci. U S A 115, 2478–2483. https://doi.org/10.1073/pnas.1714717115 (2018).
Cloutier, M. M. et al. Managing asthma in adolescents and adults: 2020 asthma guideline update from the national asthma education and prevention program. JAMA 324, 2301–2317. https://doi.org/10.1001/jama.2020.21974 (2020).
Pan, M. et al. Shenling Baizhu powder alleviates non-alcoholic fatty liver disease by modulating autophagy and energy metabolism in high-fat diet-induced rats. Phytomedicine 130, 155712. https://doi.org/10.1016/j.phymed.2024.155712 (2024).
Gao, Z. et al. Exploration the mechanism of Shenling Baizhu San in the treatment of chronic obstructive pulmonary disease based on UPLC-Q-TOF-MS/MS, network pharmacology and in vitro experimental verification. J. Ethnopharmacol. 324, 117728. https://doi.org/10.1016/j.jep.2024.117728 (2024).
Lambrecht, B. N. & Hammad, H. The immunology of asthma. Nat. Immunol. 16, 45–56. https://doi.org/10.1038/ni.3049 (2015).
Lu, C. et al. High humidity and NO(2) co-exposure exacerbates allergic asthma by increasing oxidative stress, inflammatory and TRP protein expressions in lung tissue. Environ. Pollut. 353, 124127. https://doi.org/10.1016/j.envpol.2024.124127 (2024).
Thomas, R., Qiao, S. & Yang, X. Th17/Treg imbalance: Implications in lung inflammatory diseases. Int. J. Mol. Sci. https://doi.org/10.3390/ijms24054865 (2023).
Barcik, W., Boutin, R. C. T., Sokolowska, M. & Finlay, B. B. The role of lung and gut microbiota in the pathology of asthma. Immunity 52, 241–255. https://doi.org/10.1016/j.immuni.2020.01.007 (2020).
Hoang, T. K. et al. Protective effect of Lactobacillus reuteri DSM 17938 against experimental necrotizing enterocolitis is mediated by Toll-like receptor 2. Am. J. Physiol. Gastrointest. Liver Physiol. 315, G231-g240. https://doi.org/10.1152/ajpgi.00084.2017 (2018).
Liu, Y., Tran, D. Q., Fatheree, N. Y. & Marc Rhoads, J. Lactobacillus reuteri DSM 17938 differentially modulates effector memory T cells and Foxp3+ regulatory T cells in a mouse model of necrotizing enterocolitis. Am. J. Physiol. Gastrointest. Liver Physiol. 307, G177-186. https://doi.org/10.1152/ajpgi.00038.2014 (2014).
Luo, Z. et al. Limosilactobacillus reuteri in immunomodulation: Molecular mechanisms and potential applications. Front. Immunol. 14, 1228754. https://doi.org/10.3389/fimmu.2023.1228754 (2023).
Sim, S., Park, H. J., Kim, Y. K., Choi, Y. & Park, H. S. Lactobacillus paracasei-derived extracellular vesicles alleviate neutrophilic asthma by inhibiting the JNK pathway in airway epithelium. Allergol. Int. 73, 302–312. https://doi.org/10.1016/j.alit.2023.10.008 (2024).
Uwaezuoke, S. N. et al. Postnatal probiotic supplementation can prevent and optimize treatment of childhood asthma and atopic disorders: A systematic review of randomized controlled trials. Front. Pediatr. 10, 956141. https://doi.org/10.3389/fped.2022.956141 (2022).
Drago, L. et al. The probiotics in pediatric asthma management (PROPAM) study in the primary care setting: A randomized, controlled, double-blind trial with Ligilactobacillus salivarius LS01 (DSM 22775) and Bifidobacterium breve B632 (DSM 24706). J. Immunol. Res. 2022, 3837418. https://doi.org/10.1155/2022/3837418 (2022).
Yang, M. et al. Effects of pear extracts on microbiome and immunocytokines to alleviate air pollution-related respiratory hypersensitivity. J. Med. Food 26, 211–214. https://doi.org/10.1089/jmf.2022.K.0117 (2023).
Rahal, Z. et al. Inflammation mediated by gut microbiome alterations promotes lung cancer development and an immunosuppressed tumor microenvironment. Cancer Immunol. Res. https://doi.org/10.1158/2326-6066.Cir-24-0469 (2024).
Fu, J. et al. Gut commensal Alistipes as a potential pathogenic factor in colorectal cancer. Discov. Oncol. 15, 473. https://doi.org/10.1007/s12672-024-01393-3 (2024).
Li, C., Deng, L., Pu, M., Ye, X. & Lu, Q. Coptisine alleviates colitis through modulating gut microbiota and inhibiting TXNIP/NLRP3 inflammasome. J. Ethnopharmacol. 335, 118680. https://doi.org/10.1016/j.jep.2024.118680 (2024).
Yang, X. et al. Total alkaloids of Aconitum carmichaelii Debx alleviate cisplatin-induced acute renal injury by inhibiting inflammation and oxidative stress related to gut microbiota metabolism. Phytomedicine 135, 156128. https://doi.org/10.1016/j.phymed.2024.156128 (2024).
Vital, M., Howe, A. C. & Tiedje, J. M. Revealing the bacterial butyrate synthesis pathways by analyzing (meta)genomic data. MBio 5, e00889. https://doi.org/10.1128/mBio.00889-14 (2014).
Liu, H. et al. Sishen Pill & Tongxieyaofang ameliorated ulcerative colitis through the activation of HIF-1alpha acetylation by gut microbiota-derived propionate and butyrate. Phytomedicine 136, 156264. https://doi.org/10.1016/j.phymed.2024.156264 (2025).
Parada Venegas, D. et al. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 10, 277. https://doi.org/10.3389/fimmu.2019.00277 (2019).
Mohammed, V., Muthuramamoorthy, M., Arasu, M. V. & Arockiaraj, J. Gut microbiome and mitochondrial crosstalk in Schizophrenia, a mental disability: Emerging mechanisms and therapeutic targets. Neurosci. Biobehav. Rev. 178, 106371. https://doi.org/10.1016/j.neubiorev.2025.106371 (2025).
Yang, J. et al. Lung-gut microbiota and tryptophan metabolites changes in neonatal acute respiratory distress syndrome. J. Inflamm. Res. 17, 3013–3029. https://doi.org/10.2147/JIR.S459496 (2024).
Cercamondi, C. I. et al. A postbiotic derived from lactobacillaceae protects intestinal barrier function in a challenge model using colon organoid tubules. Foods https://doi.org/10.3390/foods14071173 (2025).
De Filippis, F., Pasolli, E. & Ercolini, D. The food-gut axis: Lactic acid bacteria and their link to food, the gut microbiome and human health. FEMS Microbiol. Rev. 44, 454–489. https://doi.org/10.1093/femsre/fuaa015 (2020).
Wu, Y. et al. Methionine ameliorates intestinal injury in senescence-accelerated mouse prone-8 mice by reducing sulfate-reducing bacteria and enhancing barrier function. Front. Nutr. 12, 1698518. https://doi.org/10.3389/fnut.2025.1698518 (2025).
Li, H. et al. Transcriptome and metabolome analyses reveal the mechanisms by which H(2)S improves energy and nitrogen metabolism in tall fescue under low-light stress. Physiol. Plant 176, e70015. https://doi.org/10.1111/ppl.70015 (2024).
Liang, T. et al. Lactiplantibacillus plantarum strain 84–3-derived l-glutamine ameliorates glucose homeostasis via AMPK/PPARgamma signaling pathway activation in type 2 diabetes. Metabolism 172, 156357. https://doi.org/10.1016/j.metabol.2025.156357 (2025).
Xu, Y. et al. Fecal microbiota transplantation improves growth performance of chickens by increasing the intestinal Lactobacillus and glutamine. Poult. Sci. 104, 105243. https://doi.org/10.1016/j.psj.2025.105243 (2025).
Saito, H. et al. Omics in allergy and asthma. J. Allergy Clin. Immunol. https://doi.org/10.1016/j.jaci.2024.09.023 (2024).
Chang, C. J. et al. Metabolomics identifies metabolite markers in plasma and extracellular vesicles within plasma in patients with asthma. Clin. Chim. Acta 565, 120010. https://doi.org/10.1016/j.cca.2024.120010 (2024).
Kim, H. K., Song, C. H., Bae, Y. S., Im, S. Y. & Lee, H. K. Glutamine prevents late-phase anaphylaxis via MAPK phosphatase 1-dependent cytosolic phospholipase A2 deactivation. Int. Arch Allergy Immunol. 171, 61–70. https://doi.org/10.1159/000452103 (2016).
Kim, J. M. et al. Glutamine deficiency shifts the asthmatic state toward neutrophilic airway inflammation. Allergy 77, 1180–1191. https://doi.org/10.1111/all.15121 (2022).
Milanović, M. et al. Exogenous α-ketoglutarate modulates redox metabolism and functions of human dendritic cells, altering their capacity to polarise T cell response. Int. J. Biol. Sci. 20, 1064–1087. https://doi.org/10.7150/ijbs.91109 (2024).
Yang, L. et al. Bergenin, a PPARγ agonist, inhibits Th17 differentiation and subsequent neutrophilic asthma by preventing GLS1-dependent glutaminolysis. Acta Pharmacol. Sin. 43, 963–976. https://doi.org/10.1038/s41401-021-00717-1 (2022).
Xu, T. et al. Metabolic control of T(H)17 and induced T(reg) cell balance by an epigenetic mechanism. Nature 548, 228–233. https://doi.org/10.1038/nature23475 (2017).
Johnson, M. O. et al. Distinct regulation of Th17 and Th1 cell differentiation by glutaminase-dependent metabolism. Cell 175, 1780-1795.e1719. https://doi.org/10.1016/j.cell.2018.10.001 (2018).
Zelena, E. et al. Development of a robust and repeatable UPLC-MS method for the long-term metabolomic study of human serum. Anal. Chem. 81, 1357–1364. https://doi.org/10.1021/ac8019366 (2009).
Nair, A., Morsy, M. A. & Jacob, S. Dose translation between laboratory animals and human in preclinical and clinical phases of drug development. Drug. Dev. Res. 79, 373–382. https://doi.org/10.1002/ddr.21461 (2018).
Liu, Z. et al. Cordycepin mitigates dextran sulfate sodium-induced colitis through improving gut microbiota composition and modulating Th1/Th2 and Th17/Treg balance. Biomed. Pharmacother. 180, 117394. https://doi.org/10.1016/j.biopha.2024.117394 (2024).
Liu, X. et al. A systematic pharmacology-based in vivo study to reveal the effective mechanism of Yupingfeng in asthma treatment. Phytomedicine 114, 154783. https://doi.org/10.1016/j.phymed.2023.154783 (2023).
Li, H. et al. Gleditsiae Sinensis Fructus ingredients and mechanism in anti-asthmatic bronchitis research. Phytomedicine 133, 155857. https://doi.org/10.1016/j.phymed.2024.155857 (2024).
Kanehisa, M. et al. From genomics to chemical genomics: New developments in KEGG. Nucleic Acids Res. 34, D354-357. https://doi.org/10.1093/nar/gkj102 (2006).
Powell, S. et al. eggNOG v4.0: Nested orthology inference across 3686 organisms. Nucleic Acids Res. 42, D231-239. https://doi.org/10.1093/nar/gkt1253 (2014).
Cantarel, B. L. et al. The Carbohydrate-Active EnZymes database (CAZy): an expert resource for Glycogenomics. Nucleic Acids Res. 37, D233-238. https://doi.org/10.1093/nar/gkn663 (2009).
Kanehisa, M. & Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30. https://doi.org/10.1093/nar/28.1.27 (2000).
Funding
Doctoral Scientific Initiate Project of Shunde Women and Children’s Hospital of Guangdong Medical University (Maternity & Child Healthcare Hospital of Shunde Foshan) (2023BSQD002); The Project of Administration of Traditional Chinese Medicine of Guangdong Province (20241090); Guangzhou Municipal Science and Technology Project (2023A04J0550); Medical Research Project of Foshan Municipal Health Bureau (20250403).
Author information
Authors and Affiliations
Contributions
Yuning Zeng contributed to the design, methodology, validation and manuscript drafting. Hui Qi and Weijian Guo analyzed the data and drafted the manuscript. Xueyi Tan collected the data and prepared the figures and tables. Bo Huang and Ruihui Hu conducted the statistical analysis. Xueren Ouyang supervised the study and contributed to the design and review of a manuscript. All authors approved the submitted version and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The study is reported in accordance with ARRIVE guidelines. Animal study procedures were followed by the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Experimental Animal Ethics Committee of Guangzhou University of Chinese Medicine (No. 20240229024).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zeng, Y., Qi, H., Guo, W. et al. Multi-omics insights into Shenling Baizhu Powder’s amelioration of murine asthma through gut microbiota and Glutamine-GLS1 pathway. Sci Rep (2026). https://doi.org/10.1038/s41598-026-38440-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-38440-8