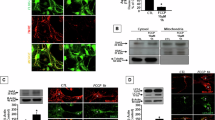
Abstract
Pancortin (PCT), a protein highly expressed in the cortex during neurodevelopment, comprises four isoforms (PCT1–4) characterized by a central B-region and N-termini (A1/A2) and C-termini (C1/C2). While PCT2, enriched in adult mouse cortex, mediates ischemic neuronal death via interactions with WAVE1 and Bcl-xL, perinatal isoforms (A2-PCTs, namely PCT3 and PCT4) remain poorly defined. Given the vulnerability of the developing brain to hypoxia-induced neurological deficits, here we asked whether A2-PCTs contribute to ischemic damage in the developing cortex. In primary cortical neurons, knockdown of PCTs mitigated cell death induced by oxygen-glucose deprivation. Consistently, using A2-PCTs knockout mice subjected to middle cerebral artery occlusion, we observed significantly reduced cortical damage in younger animals, implicating A2-PCTs as pro-death factors in hypoxic conditions. Mechanistically, A2-PCTs formed a tripartite complex with Bcl-xL and WAVE1. Overexpression of A2-PCTs in HEK293 cells resulted in elevated cell mortality, and as revealed by SPLICS (a split-GFP-based contact site sensor)-based imaging of mitochondria-ER contact sites (MERCs), increased its localization to MERCs. Furthermore, A2-PCTs interacted with GRP75, a MERCs-tethering protein, in a Bcl-xL/WAVE1-dependent manner. A2-PCTs/Bcl-xL/WAVE1 complex increased IP3R-mediated calcium transfer from the ER to mitochondria, leading to cytosolic and mitochondrial calcium overload, which was attenuated by IP3R inhibition. In Neuro2a cells subjected to oxygen-glucose deprivation, PCTs knockdown similarly suppressed pathological calcium flux. Our study identifies A2-PCTs as key regulators of MERCs-mediated calcium dysregulation in neonatal stroke, pointing to their potential as therapeutic targets for mitigating ischemic brain injury in the developing brain.
Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Huang, G. et al. Bioactive nanoenzyme reverses oxidative damage and Endoplasmic reticulum stress in neurons under ischemic stroke. ACS Nano. 16, 431–452 (2022).
Hilkens, N. A., Casolla, B., Leung, T. W. & de Leeuw, F. E. Stroke Lancet 403, 2820–2836 (2024).
Bonkhoff, A. K. et al. Outcome after acute ischemic stroke is linked to sex-specific lesion patterns. Nat. Commun. 12, 3289 (2021).
Barthels, D. & Das, H. Current advances in ischemic stroke research and therapies. Biochim. Biophys. Acta Mol. Basis Dis. 1866, 666–676 (2020).
Tu, W. J. et al. China stroke surveillance report 2021. Mil Med. Res. 10, 33 (2023).
Zhang, Y., Zhang, G. & Chen, X. Elevated calcium after acute ischemic stroke predicts severity and prognosis. Mol. Neurobiol. 61, 266–275 (2024).
Dunbar, M. & Kirton, A. Perinatal stroke: mechanisms, management, and outcomes of early cerebrovascular brain injury. Lancet Child. Adolesc. Health. 2, 666–676 (2018).
Barembaum, M., Moreno, T. A., LaBonne, C., Sechrist, J. & Bronner-Fraser, M. Noelin-1 is a secreted glycoprotein involved in generation of the neural crest. Nat. Cell. Biol. 2, 219–225 (2000).
Yokoe, H. & Anholt, R. R. H. Molecular cloning of olfactomedin, an extracellular matrix protein specific to olfactory neuroepithelium. Proc. Natl. Acad. Sci. U S A. 90, 4655–4659 (1993).
Nagano, T. et al. Differentially expressed olfactomedin-related glycoproteins Pancortins in the brain. Mol. Brain Res. 53, 13–23 (1998).
Nagano, T. et al. A2-pancortins (Pancortin-3 and – 4) are the dominant Pancortins during neocortical development. J. Neurochem. 75, 1–8 (2000).
Ando, K. et al. Expression and characterization of disulfide bond use of oligomerized A2-Pancortins: extracellular matrix constituents in the developing brain. Neuroscience 133, 947–957 (2005).
Cheng, A. et al. Pancortin-2 interacts with WAVE1 and Bcl-xL in a Mitochondria-Associated protein complex that mediates ischemic neuronal death. J. Neurosci. 27, 1519–1528 (2007).
Shah, K. & Rossie, S. Tale of the good and the bad Cdk5: remodeling of the actin cytoskeleton in the brain. Mol. Neurobiol. 55, 3426–3438 (2018).
Kang, R. et al. WAVE1 regulates Bcl-2 localization and phosphorylation in leukemia cells. Leukemia 24, 177–186 (2010).
Yang, J., Li, J. H., Wang, J. & Zhang, C. Y. Molecular modeling of BAD complex resided in a mitochondrion integrating Glycolysis and apoptosis. J. Theor. Biol. 266, 231–241 (2010).
Szabadkai, G. et al. Chaperone-mediated coupling of Endoplasmic reticulum and mitochondrial Ca2 + channels. J. Cell. Biol. 175, 901–911 (2006).
Monaco, G. et al. The BH4 domain of Anti-apoptotic Bcl-XL, but not that of the related Bcl-2, limits the Voltage-dependent anion channel 1 (VDAC1)-mediated transfer of Pro-apoptotic Ca2 + Signals to mitochondria. J. Biol. Chem. 290, 9150–9161 (2015).
Lv, S., Geng, X., Yun, H. J. & Ding, Y. Phenothiazines reduced autophagy in ischemic stroke through Endoplasmic reticulum (ER) stress-associated PERK-eIF2α pathway. Exp. Neurol. 369 https://doi.org/10.1016/j.expneurol.2023.114524 (2023).
Popgeorgiev, N., Jabbour, L. & Gillet, G. Subcellular localization and dynamics of the Bcl-2 family of proteins. Front. Cell. Dev. Biol. 6 https://doi.org/10.3389/fcell.2018.00013 (2018).
Kaufmann, T. et al. Characterization of the signal that directs Bcl-x(L), but not Bcl-2, to the mitochondrial outer membrane. J. Cell. Biol. 160, 53–64 (2003).
Cieri, D. et al. SPLICS: A split green fluorescent protein-based contact site sensor for narrow and wide heterotypic organelle juxtaposition. Cell. Death Differ. 25, 1131–1145 (2018).
Vallese, F. et al. An expanded palette of improved SPLICS reporters detects multiple organelle contacts in vitro and in vivo. Nat. Commun. 11 https://doi.org/10.1038/s41467-020-19892-6 (2020).
Pronker, M. F., Hugo, V. D. H. & Janssen, B. J. C. Design and structural characterisation of olfactomedin-1 variants as tools for functional studies. BMC Mol. Cell. Biol. 20 https://doi.org/10.1186/s12860-019-0232-1 (2019).
Poston, C. N. & Krishnan, S. C. Bazemore-Walker, C. R. In-depth proteomic analysis of mammalian mitochondria-associated membranes (MAM). J. Proteom. 79, 219–230 (2013).
Soderling, S. H. et al. A WAVE-1 and WRP signaling complex regulates spine density, synaptic plasticity, and memory. J. Neurosci. 27, 355–365 (2007).
Kim, Y. et al. Phosphorylation of WAVE1 regulates actin polymerization and dendritic spine morphology. Nature 442, 814–817 (2006).
Lebensohn, A. M. & Kirschner, M. W. Activation of the WAVE complex by coincident signals controls actin assembly. Mol. Cell. 36, 512–524 (2009).
Sossey-Alaoui, K., Head, K., Nowak, N. & Cowell, J. K. Genomic organization and expression profile of the human and mouse WAVE gene family. Mamm. Genome. 14, 314–322 (2003).
Danial, N. N. et al. BAD and glucokinase reside in a mitochondrial complex that integrates Glycolysis and apoptosis. Nature 424, 952–956 (2003).
Sarmah, D. et al. Mitochondrial dysfunction in stroke: implications of stem cell therapy. Transl Stroke Res. 10, 121–136 (2019).
Chen, X. et al. GRP75 triggers white adipose tissue Browning to promote cancer-associated cachexia. Signal. Transduct. Target. Ther. 9 https://doi.org/10.1038/s41392-024-01950-w (2024).
Gu, J. et al. Advances in the structures, mechanisms and targeting of molecular chaperones. Signal. Transduct. Target. Ther. 10 https://doi.org/10.1038/s41392-025-02166-2 (2025).
Rosario et al. Close Contacts with the Endoplasmic Reticulum as Determinants of Mitochondrial Ca2 + Responses Responses. Science 280, 1763–1766 (1998). (1979).
Giorgi, C., Marchi, S. & Pinton, P. The machineries, regulation and cellular functions of mitochondrial calcium. Nat. Rev. Mol. Cell. Biol. 19, 713–730 (2018).
Pivovarova, N. B. & Andrews, S. B. Calcium-dependent mitochondrial function and dysfunction in neurons. FEBS J. 277, 3622–3636 (2010).
Bodalia, A., Li, H. & Jackson, M. F. Loss of Endoplasmic reticulum Ca2 + homeostasis: contribution to neuronal cell death during cerebral ischemia. Acta Pharmacol. Sin. 34, 49–59 (2013).
Kelley, N., Jeltema, D., Duan, Y. & He, Y. The NLRP3 inflammasome: an overview of mechanisms of activation and regulation. Int. J. Mol. Sci. 20, 3328–3352 (2019).
Ciscato, F. et al. Hexokinase 2 displacement from mitochondria-associated membranes prompts Ca 2+ ‐dependent death of cancer cells. EMBO Rep. 21, e49117. https://doi.org/10.15252/embr.201949117 (2020).
Baik, S. H. et al. Hexokinase dissociation from mitochondria promotes oligomerization of VDAC that facilitates NLRP3 inflammasome assembly and activation. Sci. Immunol. 8, eade7652 (2023).
Moffat, J. et al. A lentiviral RNAi library for human and mouse genes applied to an arrayed viral High-Content screen. Cell 124, 1283–1298 (2006).
Iguchi, T. et al. Mutually repulsive EphA7–EfnA5 organize region-to-region corticopontine projection by inhibiting collateral extension. J. Neurosci. 41, 4795–4808 (2021).
Taguchi, A. et al. A reproducible and simple model of permanent cerebral ischemia in CB-17 and SCID mice. J. Exp. Stroke Transl Med. 3, 28–33 (2010).
Kasahara, Y. et al. A highly reproducible model of cerebral ischemia/reperfusion with extended survival in CB-17 mice. Neurosci. Res. 76, 163–168 (2013).
Niwa, H., Yamamura, K. I. & Miyazaki, J. I. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene 108, 193–200 (1991).
Sridharan, S. et al. High-performance microbial Opsins for spatially and temporally precise perturbations of large neuronal networks. Neuron 110, 1139–1155e6 (2022).
Suzuki, J. et al. Imaging intraorganellar Ca2 + at subcellular resolution using CEPIA. Nat. Commun. 5, 4153 (2014).
Larrañaga-SanMiguel, A., Bengoa-Vergniory, N. & Flores-Romero, H. Crosstalk between mitochondria–ER contact sites and the apoptotic machinery as a novel health meter. Trends Cell. Biol. 35, 33–45 (2025).
Acknowledgements
We extend our sincere gratitude to all members of the research team for their invaluable contributions and collaborative efforts throughout this study, especially those who worked for this project at the beginning, such as Takashi Nagano, Mitsuyo Maeda, and Hiroshi Takagi. We thank the Genome Information Research Center, Research Institute for Microbial Diseases (RIMD), The University of Osaka, for their assistance in generating the knockout mice. We would like to express our gratitude to the Center for Medical Research and Education (CentMERe), The University of Osaka, for providing access to and assistance with microscopy services. We also thank The Center of Medical Innovation and Translational Research (CoMIT) for performing the sequencing and for their support with the OGD experiments. Their contributions were essential to this work. We thank Jia-Xuan Li for assistance with Western blotting, Ming-Yue Ma for establishing and maintaining neuronal cultures, Guo-Qiang Yang and Zhen-Ying Zhang for quantitative analysis of cell death, and Nikita Diubachev, Lan Yang, and Ghita Bennis for quantification of cellular staining signals.
Funding
This work was supported in part by Naito Foundation, Senri-Life Science Foundation, and the Ministry of Education, Science, Sports and Culture of Japan (to M.S., grand IDs: 20H03414, 15K15015, 25293043, 19390048, 11878150, 07780686).
Author information
Authors and Affiliations
Contributions
Q.Y.: methodology, investigation (experimental design and execution), formal analysis, resources, data curation, original manuscript; C.W.: methodology (molecular engineering), resources (plasmid constructs: pCAGGS-PCT, pCAGGS-WAVE1, pCAGGS-Bcl-xL), formal analysis (MCAO statistical datasets); M.X., T.M.: investigation (MCAO surgery); H.Y.: methodology (murine knockout models), manuscript revision, project administration; M.Y.: manuscript revision, supervision, discussion, validation, methodology (technical guidance); C.T., Y.O.: manuscript revision, methodology (technical guidance), supervision, discussion; T.M., K.K.: project administration; M.S: project administration, manuscript revision, supervision, discussion, initial conceptualization and experimental design.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yang, Q., Wang, CC., Matsuyama, T. et al. A2-pancortins interact with Bcl-xL and WAVE1 to promote mitochondria-ER contact sites (MERCs) and exacerbate mitochondrial calcium elevation to mediate cell death in stroke. Sci Rep (2026). https://doi.org/10.1038/s41598-026-38928-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-38928-3