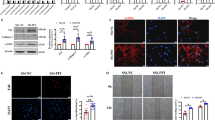
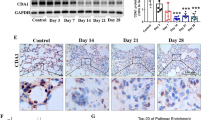
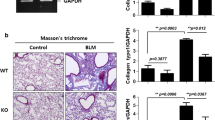
Abstract
Pulmonary fibrosis is a fatal interstitial lung disease marked by irreversible lung structure destruction. Understanding its molecular mechanisms is essential. Cold-inducible RNA-binding protein (CIRBP), a stress-responsive protein, stabilizes mRNA intracellularly and also acts extracellularly. We previously demonstrated that CIRBP is highly expressed in fibrotic lesions of idiopathic pulmonary fibrosis patients, with elevated serum levels correlating with disease progression and poor prognosis. However, its role in fibrosis remains unclear. We investigated CIRBP’s function using an in vivo bleomycin (BLM)-induced pulmonary fibrosis mouse model via intratracheal administration and an in vitro model with primary lung fibroblasts. CIRBP expression was markedly upregulated in the fibrotic regions of BLM-treated wild-type (WT) mice. CIRBP-deficient mice showed improved survival, reduced fibrosis, lower hydroxyproline content, and decreased expression of α-SMA and fibronectin. Therapeutic administration of C23, a CIRBP-derived inhibitory peptide, significantly suppressed fibrosis and improved survival in WT mice. In vitro, recombinant CIRBP promoted collagen secretion, fibroblast proliferation, and cell migration, which were inhibited by C23. Mechanistically, CIRBP-induced IL-6 production via TLR2 and TLR4, leading to fibroblast activation through autocrine signaling. These effects were suppressed by C23, TLR2/4 inhibitors, or IL-6 neutralization. Our findings identify CIRBP as a novel profibrotic factor and therapeutic target in pulmonary fibrosis.
Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request. The RNA-seq data generated in this study have been deposited in the Gene Expression Omnibus (GEO) under accession number GSE294583 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE294583). The data can be accessed with the following token: kfityyewrncrvir during peer review.
References
Raghu, G. et al. Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: An official ATS/ERS/JRS/ALAT clinical practice guideline. Am. J. Respir. Crit. Care Med. 205, e18–e47 (2022).
Rajan, S. K. et al. Progressive pulmonary fibrosis: An expert group consensus statement. Eur. Respir. J. 61, 2103187 (2023).
Popper, H., Stacher-Priehse, E., Brcic, L. & Nerlich, A. Lung fibrosis in autoimmune diseases and hypersensitivity: How to separate these from idiopathic pulmonary fibrosis. Rheumatol. Int. 42, 1321–1330 (2022).
Lederer, D. J. & Martinez, F. J. Idiopathic pulmonary fibrosis. N. Engl. J. Med. 378, 1811–1823 (2018).
Wijsenbeek, M. & Cottin, V. Spectrum of fibrotic lung diseases. N. Engl. J. Med. 383, 958–968 (2020).
Desai, O., Winkler, J., Minasyan, M. & Herzog, E. L. The role of immune and inflammatory cells in idiopathic pulmonary fibrosis. Front. Med. (Lausanne) 5, 43 (2018).
Ellson, C. D., Dunmore, R., Hogaboam, C. M., Sleeman, M. A. & Murray, L. A. Danger-associated molecular patterns and danger signals in idiopathic pulmonary fibrosis. Am. J. Respir. Cell. Mol. Biol. 51, 163–168 (2014).
Corre, M. & Lebreton, A. Regulation of cold-inducible RNA-binding protein (CIRBP) in response to cellular stresses. Biochimie 217, 3–9 (2024).
Liao, Y., Tong, L., Tang, L. & Wu, S. The role of cold-inducible RNA binding protein in cell stress response. Int. J. Cancer 141, 2164–2173 (2017).
Zhong, P. et al. The role of Cold-Inducible RNA-binding protein in respiratory diseases. J. Cell. Mol. Med. 26, 957–965 (2022).
Hozumi, H. et al. Clinical significance of cold-inducible RNA-binding protein in idiopathic pulmonary fibrosis. Chest 160, 2149–2157 (2021).
Bolourani, S., Sari, E., Brenner, M. & Wang, P. The role of eCIRP in bleomycin-induced pulmonary fibrosis in mice. PLoS ONE 17, e0266163 (2022).
Jenkins, R. G. et al. An Official American Thoracic Society workshop report: Use of animal models for the preclinical assessment of potential therapies for pulmonary fibrosis. Am. J. Respir. Cell. Mol. Biol. 56, 667–679 (2017).
Masuda, T. et al. Cold-inducible RNA-binding protein (Cirp) interacts with Dyrk1b/Mirk and promotes proliferation of immature male germ cells in mice. Proc. Natl. Acad. Sci. U.S.A. 109, 10885–10890 (2012).
Qiang, X. et al. Cold-inducible RNA-binding protein (CIRP) triggers inflammatory responses in hemorrhagic shock and sepsis. Nat. Med. 19, 1489–1495 (2013).
Ashcroft, T., Simpson, J. M. & Timbrell, V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J. Clin. Pathol. 41, 467–470 (1988).
Bolourani, S., Sari, E., Brenner, M. & Wang, P. Extracellular CIRP induces an inflammatory phenotype in pulmonary fibroblasts via TLR4. Front. Immunol. 12, 721970 (2021).
Nishiyama, H. et al. A glycine-rich RNA-binding protein mediating cold-inducible suppression of mammalian cell growth. J. Cell. Biol. 137, 899–908 (1997).
Danno, S. et al. Increased transcript level of RBM3, a member of the glycine-rich RNA-binding protein family, in human cells in response to cold stress. Biochem. Biophys. Res. Commun. 236, 804–807 (1997).
Xue, J. H. et al. Effects of ischemia and H2O2 on the cold stress protein CIRP expression in rat neuronal cells. Free Radic. Biol. Med. 27, 1238–1244 (1999).
Qu, Y. & Dubyak, G. R. P2X7 receptors regulate multiple types of membrane trafficking responses and non-classical secretion pathways. Purinergic Signal 5, 163–173 (2009).
Denning, N. L. et al. Extracellular CIRP as an endogenous TREM-1 ligand to fuel inflammation in sepsis. JCI Insight 5, e134172 (2020).
She, Y. X., Yu, Q. Y. & Tang, X. X. Role of interleukins in the pathogenesis of pulmonary fibrosis. Cell Death Discov. 7, 52 (2021).
Li, Y. et al. The role of IL-6 in fibrotic diseases: Molecular and cellular mechanisms. Int. J. Biol. Sci. 18, 5405–5414 (2022).
Duncan, M. R. & Berman, B. Stimulation of collagen and glycosaminoglycan production in cultured human adult dermal fibroblasts by recombinant human interleukin 6. J. Invest. Dermatol. 97, 686–692 (1991).
Le, T. T. et al. Blockade of IL-6 Trans signaling attenuates pulmonary fibrosis. J. Immunol. 193, 3755–3768 (2014).
Fries, K. M., Felch, M. E. & Phipps, R. P. Interleukin-6 is an autocrine growth factor for murine lung fibroblast subsets. Am. J. Respir. Cell. Mol. Biol. 11, 552–560 (1994).
Narazaki, M. & Kishimoto, T. Current status and prospects of IL-6-targeting therapy. Expert Rev. Clin. Pharmacol. 15, 575–592 (2022).
Hunter, C. A. & Jones, S. A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 16, 448–457 (2015).
Molyneaux, P. L. & Maher, T. M. The role of infection in the pathogenesis of idiopathic pulmonary fibrosis. Eur. Respir. Rev. 22, 376–381 (2013).
Raghu, G., Anstrom, K. J., King, T. E. Jr., Lasky, J. A. & Martinez, F. J. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N. Engl. J. Med. 366, 1968–1977 (2012).
Acknowledgements
The authors thank Enago (www.enago.jp) for the English language review. Part of this work was conducted at the Advanced Research Facilities & Services, Hamamatsu University School of Medicine. We sincerely thank Asuka Miyagi and Ryo Horiguchi for their invaluable assistance.
Funding
This study was supported by a grant from the Japan Society for the Promotion of Science (21K08176 to H.H.), a grant from the TERUMO LIFE SCIENCE FOUNDATION (22-III 4014 to H.H.), and a HUSM Grant-in-Aid. The funding source did not provide any input or contributions in the development of the research or manuscript.
Author information
Authors and Affiliations
Contributions
YM: Conception and design, data collection, data analysis and interpretation, manuscript writing. HH: Conception and design, data collection, data analysis and interpretation, manuscript writing, and final approval of the manuscript. AF, HW, HN, YI, HY, YS, MK, KF, NE, TF and NI: Data collection, data analysis, and supervision. TS: Conception and design, manuscript writing, and administrative support.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Declaration of Generative AI and AI-assisted technologies in the writing process
None of Generative AI and AI-assisted technologies were used in this manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mochizuka, Y., Hozumi, H., Watanabe, H. et al. Cold inducible RNA binding protein promotes fibroblast activation and its inhibition represents a potential therapeutic target in pulmonary fibrosis. Sci Rep (2026). https://doi.org/10.1038/s41598-026-39649-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-39649-3