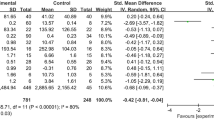
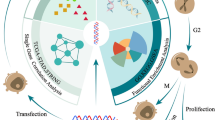
Abstract
This study aimed to investigate the impact of chronic inflammation (CI) on gastric cancer (GC) progression and the underlying molecular mechanisms. A subcutaneous xenograft model using 615-strain mice was established to evaluate the pro-tumorigenic effects of CI. Proteome Profiler Mouse XL Cytokine Array was used to screen for key pro-tumorigenic cytokines induced by CI, and ELISA was employed for validation. single-cell RNA sequencing(ScRNA-seq) was performed to identify the cellular source of CX3CL1 in GC tumor tissues. CCK-8 and colony formation assays were used to assess the effect of CX3CL1 on GC cell proliferation, while wound-healing and Transwell assays evaluated cell migration. ADAM10 expression was measured in gastric cancer cells and tissues via qRT-PCR, Western blot, and immunofluorescence staining. CI significantly accelerated gastric cancer progression. CX3CL1 expression was markedly higher in GC tissues than in normal gastric tissues, and high CX3CL1 expression was associated with poor prognosis in GC patients. CX3CL1 recombinant protein significantly promoted the proliferation and migration of GC cells, and these effects were attenuated by pharmacological inhibition of CX3CR1. Mechanistically, CI upregulated the expression of ADAM10, which plays a key role in converting membrane-bound CX3CL1 to its soluble form. This study provided evidence that chronic inflammation could promote tumor progression through the activation of ADAM10/CX3CL1 axis in gastric cancer.
Similar content being viewed by others
Data availability
The single-cell RNA-seq raw sequencing data have been deposited in the NCBI Sequence Read Archive (SRA, an INSDC member repository) with accessions SRR37061229 and SRR37061230. BioProject: PRJNA1416616; BioSamples: SAMN54972753 and SAMN54972754. The processed single-cell expression matrices have been deposited in the Gene Expression Omnibus (GEO) under accession number GSE318425.
References
Bray, F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 74, 229–263 (2024).
Smyth, E. C. et al. Gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 27, v38–v49 (2016).
Hanahan, D. Hallmarks of cancer: new dimensions. Cancer Discov. 12, 31–46 (2022).
Coorens, T. H. H. et al. The somatic mutation landscape of normal gastric epithelium. Nature 640, 418–426 (2025).
Merchant, J. L. Inflammation, atrophy, gastric cancer: connecting the molecular Dots. Gastroenterology 129, 1079–1082 (2005).
Li, T. et al. Gastric cancer cell proliferation and survival is enabled by a Cyclophilin B/STAT3/miR-520d-5p signaling feedback loop. Cancer Res. 77, 1227–1240 (2017).
Chochi, K. et al. Helicobacter pylori augments growth of gastric cancers via the lipopolysaccharide-Toll-like receptor 4 pathway whereas its lipopolysaccharide attenuates antitumor activities of human mononuclear cells. Clin. Cancer Res. 14, 2909–2917 (2008).
Li, D. et al. Hypermethylation-mediated HNF4A Silencing by Helicobacter pylori infection drives gastric cancer by disrupting epithelial cell Polarity and activating EMT signaling. Cell. Death Dis. 16, 688 (2025).
Li, D. et al. Heterogeneity and plasticity of epithelial-mesenchymal transition (EMT) in cancer metastasis: focusing on partial EMT and regulatory mechanisms. Cell. Prolif. 56, e13423 (2023).
Qin, S. et al. A novel TGFbeta/TGILR axis mediates crosstalk between cancer-associated fibroblasts and tumor cells to drive gastric cancer progression. Cell. Death Dis. 15, 368 (2024).
Ni, Y. et al. CX3CL1/CX3CR1 interaction protects against lipotoxicity-induced nonalcoholic steatohepatitis by regulating macrophage migration and M1/M2 status. Metabolism 136, 155272 (2022).
Propper, D. J. & Balkwill, F. R. Harnessing cytokines and chemokines for cancer therapy. Nat. Rev. Clin. Oncol. 19, 237–253 (2022).
Liu, P. P. et al. Neuronal cathepsin S increases neuroinflammation and causes cognitive decline via CX3CL1-CX3CR1 axis and JAK2-STAT3 pathway in aging and alzheimer’s disease. Aging Cell. e14393 https://doi.org/10.1111/acel.14393 (2024).
Yu, Z. et al. Role of ADAM10/17-Mediated cleavage of LAG3 in the impairment of immunosuppression in psoriasis. J. Invest. Dermatol. 145, 1385–1395e8 (2025).
Qian, X. et al. Deciphering the role of CX3CL1-CX3CR1 in aortic aneurysm pathogenesis: insights from Mendelian randomization and transcriptomic analyses. Front. Immunol. 15, 1383607 (2024).
Eain, H. S. et al. Double-faced CX3CL1 enhances lymphangiogenesis-dependent metastasis in an aggressive subclone of oral squamous cell carcinoma. JCI Insight. 9, e174618 (2024).
Yang, Z. et al. YTHDF2 in peritumoral hepatocytes mediates chemotherapy-induced antitumor immune responses through CX3CL1-mediated CD8 + T cell recruitment. Mol. Cancer. 23, 186 (2024).
Yao, X. et al. Expression of CX3CR1 associates with cellular migration, metastasis, and prognosis in human clear cell renal cell carcinoma. Urologic Oncology: Seminars Original Investigations. 32, 162–170 (2014).
Liu, X., Yu, Z., Li, Y. & Huang, J. CX3CL1 and its receptor CX3CR1 interact with RhoA signaling to induce Paclitaxel resistance in gastric cancer. Heliyon 10, e29100 (2024).
Su, J. et al. Lactate/GPR81 recruits regulatory T cells by modulating CX3CL1 to promote immune resistance in a highly glycolytic gastric cancer. Oncoimmunology 13, 2320951 (2024).
Bazan, J. F. et al. A new class of membrane-bound chemokine with a CX3C motif. Nature 385, 640–644 (1997).
Gunner, G. et al. Sensory lesioning induces microglial synapse elimination via ADAM10 and fractalkine signaling. Nat. Neurosci. 22, 1075–1088 (2019).
Klapproth, E. et al. Targeting cardiomyocyte ADAM10 ectodomain shedding promotes survival early after myocardial infarction. Nat. Commun. 13, 7648 (2022).
Smith, T. M., Tharakan, A. & Martin, R. K. Targeting ADAM10 in cancer and autoimmunity. Front. Immunol. 11, 499 (2020).
Cao, T. et al. Cancer SLC6A6-mediated taurine uptake transactivates immune checkpoint genes and induces exhaustion in CD8 + T cells. Cell 187, 2288–2304e27 (2024).
Li, J. et al. Neutrophil extracellular traps induced by the hypoxic microenvironment in gastric cancer augment tumour growth. Cell. Commun. Signal. 21, 86 (2023).
Mueller, A. C. et al. Induction of ADAM10 by radiation therapy drives Fibrosis, Resistance, and Epithelial-to-Mesenchyal transition in pancreatic cancer. Cancer Res. 81, 3255–3269 (2021).
Kanehisa, M., Furumichi, M., Sato, Y., Matsuura, Y. & Ishiguro-Watanabe, M. KEGG: biological systems database as a model of the real world. Nucleic Acids Res. 53, D672–D677 (2025).
Nógrádi, B. et al. The CCL2-CCR2 axis drives neuromuscular denervation in amyotrophic lateral sclerosis. Nat. Commun. 16, 7053 (2025).
Stefanidakis, M. & Koivunen, E. Cell-surface association between matrix metalloproteinases and integrins: role of the complexes in leukocyte migration and cancer progression. Blood 108, 1441–1450 (2006).
Sasaki, R., Devhare, P., Ray, R. B. & Ray, R. Hepatitis C virus–induced tumor-initiating cancer stem–like cells activate stromal fibroblasts in a xenograft tumor model. Hepatology 66, 1766–1778 (2017).
Lv, Y. et al. Targeting intracellular MMPs efficiently inhibits tumor metastasis and angiogenesis. Theranostics 8, 2830–2845 (2018).
Gontero, P., Banisadr, S., Frea, B. & Brausi, M. Metastasis markers in bladder cancer: A review of the literature and clinical considerations. Eur. Urol. 46, 296–311 (2004).
Naessens, F. et al. CX3CL1 release during Immunogenic apoptosis is associated with enhanced anti-tumour immunity. Front. Immunol. 15, 1396349 (2024).
Jeong, J. M. et al. CX3CR1 + macrophages interact with HSCs to promote HCC through CD8 + T-cell suppression. Hepatology 82, 655–668 (2025).
Pang, L. et al. Postoperative plasmacytoid dendritic cells secrete IFNα to promote recruitment of Myeloid-Derived suppressor cells and drive hepatocellular carcinoma recurrence. Cancer Res. 82, 4206–4218 (2022).
Gao, J. H. et al. Direct interaction of platelet with tumor cell aggravates hepatocellular carcinoma metastasis by activating TLR4/ADAM10/CX3CL1 axis. Cancer Lett. 585, 216674 (2024).
Hundhausen, C. et al. Regulated shedding of transmembrane chemokines by the disintegrin and metalloproteinase 10 facilitates detachment of adherent leukocytes. J. Immunol. 178, 8064–8072 (2007).
Venkatesh, H. S. et al. Targeting neuronal activity-regulated neuroligin-3 dependency in high-grade glioma. Nature 549, 533–537 (2017).
Xie, Y. et al. Inflammation in cancer: therapeutic opportunities from new insights. Mol. Cancer. 24, 51 (2025).
Kureshi, C. T. & Dougan, S. K. Cytokines in cancer. Cancer Cell. 43, 15–35 (2025).
He, C. et al. Induction of CX3CL1 expression by LPS and its impact on invasion and migration in oral squamous cell carcinoma. Front. Cell. Dev. Biol. 12, 1371323 (2024).
Li, Z. et al. Macrophage DHX34 as a negative regulator of the CX3CL1-CX3CR1 axis and CD8 + T-cell infiltration in hepatocellular carcinoma. Int. Immunopharmacol. 169, 116014 (2025).
Salam, S. M. A. et al. Brain metastasis-associated cancer fibroblasts drive tumor progression and therapeutic resistance through IL26 and CX3CL1 signaling in non-small-cell lung cancer. Exp. Hematol. Oncol. 14, 120 (2025).
Kim, H. S. et al. Directly reprogrammed NK cells driven by BCL11B depletion enhance targeted immunotherapy against pancreatic ductal adenocarcinoma. J. Hematol. Oncol. 18, 100 (2025).
Seitz, S. et al. The chemokine CX3CL1 promotes intraperitoneal tumour growth despite enhanced T-cell recruitment in ovarian cancer. Neoplasia 60, 101130 (2025).
K, W. et al. Vertebral-specific activation of the CX3CL1/ICAM-1 signaling network mediates non-small-cell lung cancer spinal metastasis by engaging tumor cell-vertebral bone marrow endothelial cell interactions. Theranostics 11, (2021).
Liu, W. et al. Role of CX3CL1 in diseases. Arch. Immunol. Ther. Exp. (Warsz). 64, 371–383 (2016).
Acknowledgements
The authors gratefully acknowledge the colleagues from the Laboratory of “Blood Stasis andToxin” Syndrome for their technical support and constructive discussions.
Funding
This study was supported by the Key Support Project of Regional Innovation and Development Joint Fund of National Natural Science Foundation of China (U23A20499); Zhejiang Provincial Natural Science Foundation of China (No. LQ23H270013); National Natural Science Foundation of China (No. 82204950); Key project of the National Nature Foundation of China (No. 82030119); Zhejiang Chinese Medicine University Chunyan Special Fund for the development of traditional Chinese medicine (No. CY202302); State Administration of Traditional Chinese Medicine Science and Technology Department - Zhejiang Provincial Administration of Traditional Chinese Medicine joint science and technology plan key research project (GZY-ZJ-KJ-23094).
Author information
Authors and Affiliations
Contributions
TJ, ZZ designed the experiment. ZS, YL and ZW conducted the experiment. ZS, ZW, SZ and JZ did the data analysis. ZS wrote the manuscript. YW, TJ and ZZ provided guidance during the experimentation phase and contributed to revising the manuscript. YW, TJ, GZ acquired funding. The authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All animal care and experimental procedures adhered to the guidelines approved by the Animal Ethics Committee of Zhejiang Chinese Medical University (License Number: 20240115-04). All methods were carried out in accordance with relevant guidelines and regulations. All methods are reported in accordance with ARRIVE guidelines.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Song, Z., Liu, Y., Wang, Z. et al. Chronic inflammation promotes gastric cancer progression via ADAM10-mediated cleavage of CX3CL1. Sci Rep (2026). https://doi.org/10.1038/s41598-026-39743-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-026-39743-6