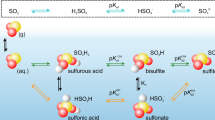
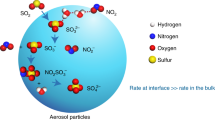
Abstract
Fast atmospheric particulate nitrate and sulfate formation under high-humidity conditions has been extensively observed; however, the underlying chemical mechanisms and their relative contributions remain poorly understood. This study examined the characteristic high-humidity events (HHEs) in southern China during spring, providing field observation evidence for the crucial role of NO2-driven multiphase reactions in particulate nitrate and sulfate formation. Our findings revealed efficient nitrate formation during early HHEs, likely facilitated by enhanced NO2 uptake via disproportionation reaction. As humidity increased and fog formed, S(IV) oxidation competitively consumed NO2 and N(III), causing rapid sulfate formation. The resulting N(III), produced from the oxidation of S(IV) by NO2 (aq), further oxidizes S(IV) effectively in droplets due to its slow liquid-gas mass transfer rate. A state-of-the-art multiphase box model demonstrated that NO2 uptake and SO2 oxidation by NO2/N(III) represent dominant formation pathways during HHEs, accounting for 45.4% and 63.6% of the total nitrate and sulfate production, respectively. These results highlight the critical importance of NO2-driven multiphase chemistry in particulate pollution under high-humidity environments.
Similar content being viewed by others
Data availability
Observed data and data analysis methods are available on request from Jinsheng Chen (jschen@iue.ac.cn).
References
Zhai, S. et al. Control of particulate nitrate air pollution in China. Nat. Geosci. 14, 389–395 (2021).
Zhao, Y. et al. Decline in bulk deposition of air pollutants in China lags behind reductions in emissions. Nat. Geosci. 15, 190–195 (2022).
Wu, X. et al. The characteristics of air pollution induced by the quasi-stationary front: formation processes and influencing factors. Sci. Total Environ. 707, 136194 (2020).
Cheng, C. et al. The significant contribution of nitrate to a severe haze event in the winter of Guangzhou, China. Sci. Total Environ. 909, 168582 (2024).
Li, H. et al. Nitrate-driven urban haze pollution during summertime over the North China Plain. Atmos. Chem. Phys. 18, 5293–5306 (2018).
Wang, G. et al. Persistent sulfate formation from London Fog to Chinese haze. Proc. Natl. Acad. Sci. USA 113, 13630–13635 (2016).
Cheng, Y. et al. Reactive nitrogen chemistry in aerosol water as a source of sulfate during haze events in China. Sci. Adv. 2, e1601530 (2016).
Wang, J. et al. Fast sulfate formation from oxidation of SO2 by NO2 and HONO observed in Beijing haze. Nat. Commun. 11, 2844 (2020).
Xu, W. et al. Efficient nitrate formation in fog events implicates fog interstitial aerosols as significant drivers of atmospheric chemistry. Environ. Sci. Technol. 58, 22298–22311 (2024).
Lee, Y. N. & Schwartz, S. E. Reaction kinetics of nitrogen dioxide with liquid water at low partial pressure. J. Phys. Chem. 85, 840–848 (1981).
Cheng, Y., Su, H., Koop, T., Mikhailov, E. & Pöschl, U. Size dependence of phase transitions in aerosol nanoparticles. Nat. Commun. 6, 5923 (2015).
Gen, M. et al. Rapid hydrolysis of NO2 at high ionic strengths of deliquesced aerosol particles. Environ. Sci. Technol. 58, 7904–7915 (2024).
Stutz, J. et al. Relative humidity dependence of HONO chemistry in urban areas. J. Geophys. Res. Atmos. 109, D03307 (2004).
Zhang, X. et al. Elucidating HONO formation mechanism and its essential contribution to OH during haze events. npj Clim. Atmos. Sci. 6, 55 (2023).
Xuan, H. et al. Relative humidity driven nocturnal HONO formation mechanism in autumn haze events of Beijing. npj Clim. Atmos. Sci. 7, 193 (2024).
Liu, T. & Abbatt, J. P. D. Oxidation of sulfur dioxide by nitrogen dioxide accelerated at the interface of deliquesced aerosol particles. Nat. Chem. 13, 1173–1177 (2021).
Li, L., Hoffmann, M. R. & Colussi, A. J. Role of nitrogen dioxide in the production of sulfate during chinese haze-aerosol episodes. Environ. Sci. Technol. 52, 2686–2693 (2018).
Wang, G. et al. Atmospheric sulfate aerosol formation enhanced by interfacial anions. PNAS Nexus. 4, 3 (2025).
Liu, L. et al. Global impact of particulate nitrate photolysis on fine sulfate aerosol. Environ. Sci. Technol. Lett. 11, 961–967 (2024).
Cho, J.-H. & Kim, H.-S. Influence of aqueous-phase chemistry on the concentrations of PM2.5 and hydrometers during the development of convective clouds over the Yellow Sea in July of 2017. J. Geophys. Res. Atmos. 129, e2023JD039421 (2024).
Hu, B. et al. Exploration of the atmospheric chemistry of nitrous acid in a coastal city of southeastern China: results from measurements across four seasons. Atmos. Chem. Phys. 22, 371–393 (2022).
Liu, T. et al. Atmospheric oxidation capacity and ozone pollution mechanism in a coastal city of southeastern China: analysis of a typical photochemical episode by an observation-based model. Atmos. Chem. Phys. 22, 2173–2190 (2022).
Chen, G. et al. Increasing contribution of chlorine chemistry to wintertime ozone formation promoted by enhanced nitrogen chemistry. Environ. Sci. Technol. 58, 22714–22721 (2024).
Brown, S. S. & Stutz, J. Nighttime radical observations and chemistry. Chem. Soc. Rev. 41, 6405–6447 (2012).
Wagner, N. L. et al. N2O5 uptake coefficients and nocturnal NO2 removal rates determined from ambient wintertime measurements. J. Geophys. Res. Atmos. 118, 9331–9350 (2013).
Schwartz S. E. Mass-transport considerations pertinent to aqueous phase reactions of gases in liquid-water clouds. in (ed. Jaeschke W.) Chemistry of Multiphase Atmospheric Systems (Springer, 1986).
Knipping, E. M. et al. Experiments and simulations of ion-enhanced interfacial chemistry on aqueous NaCl aerosols. Science 288, 301–306 (2000).
Piatkowski, L., Zhang, Z., Backus, E. H. G., Bakker, H. J. & Bonn, M. Extreme surface propensity of halide ions in water. Nat. Commun. 5, 4083 (2014).
Zhang, R. et al. Evidence on interfacial reaction governing NO2 hydrolysis in deliquesced aerosol particles. Environ. Sci. Technol. 59, 11708–11719 (2025).
Ammann, M. et al. Heterogeneous production of nitrous acid on soot in polluted air masses. Nature 395, 157–160 (1998).
Kleffmann, J., Becker, K. H. & Wiesen, P. Heterogeneous NO2 conversion processes on acid surfaces: possible atmospheric implications. Atmos. Environ. 32, 2721–2729 (1998).
Zhang, R., Gen, M., Huang, D., Li, Y. & Chan, C. K. Enhanced sulfate production by nitrate photolysis in the presence of halide ions in atmospheric particles. Environ. Sci. Technol. 54, 3831–3839 (2020).
Zhang, R. & Chan, C. K. Simultaneous formation of sulfate and nitrate via co-uptake of SO2 and NO2 by aqueous NaCl droplets: combined effect of nitrate photolysis and chlorine chemistry. Atmos. Chem. Phys. 23, 6113–6126 (2023).
Martin, L. R., Damschen, D. E. & Judeikis, H. S. The reactions of nitrogen oxides with SO2 in aqueous aerosols. Atmos. Environ. 15, 191–195 (1981).
Chang, S. G., Toossi, R. & Novakov, T. The importance of soot particles and nitrous acid in oxidizing SO2 in atmospheric aqueous droplets. Atmos. Environ. 15, 1981 (1967).
Xue, J. et al. Sulfate formation enhanced by a cocktail of high NOx, SO2, particulate matter, and droplet pH during Haze-Fog events in megacities in China: an observation-based modeling investigation. Environ. Sci. Technol. 50, 7325–7334 (2016).
Chen, H. et al. Simultaneous reduction and oxidation of NO2 on water microdroplets provides previously unknown pathways to the formation of HONO and HNO3. J. Am. Chem. Soc. 147, 38500–38507 (2025).
Ma, P. et al. Regime-dependence of nocturnal nitrate formation via N2O5 hydrolysis and its implication for mitigating nitrate pollution. Geophys. Res. Lett. 50, e2023GL106183 (2023).
Zhang, Y. et al. Double-edged role of VOCs reduction in nitrate formation: insights from observations during the China International Import Expo 2018. Environ. Sci. Technol. 57, 15979–15989 (2023).
Yang, S. et al. The formation and mitigation of nitrate pollution: comparison between urban and suburban environments. Atmos. Chem. Phys. 22, 4539–4556 (2022).
Xie, Y. et al. Nitrate-dominated PM2.5 and elevation of particle pH observed in urban Beijing during the winter of 2017. Atmos. Chem. Phys. 20, 5019–5033 (2020).
Ge, S. et al. Abundant NH3 in China enhances atmospheric HONO production by promoting the heterogeneous reaction of SO2 with NO2. Environ. Sci. Technol. 53, 14339–14347 (2019).
Lu, C. et al. Chemical composition of fog water in Nanjing area of China and its related fog microphysics. Atmos. Res. 97, 47–69 (2010).
Zhang, S. et al. Chemical characteristics of size-resolved fog water at an urban site in Nanjing and the summit of Mt. Lu, East China. Atmos. Environ. 263, 118667 (2021).
Li, P. et al. Fog water chemistry in Shanghai. Atmos. Environ. 45, 4034–4041 (2011).
Liu, Y. et al. The promotion effect of nitrous acid on aerosol formation in wintertime in Beijing: the possible contribution of traffic-related emissions. Atmos. Chem. Phys. 20, 13023–13040 (2020).
Prather, K. A. et al. Bringing the ocean into the laboratory to probe the chemical complexity of sea spray aerosol. Proc. Natl. Acad. Sci. USA 110, 7550–7555 (2013).
Collins, D. B. et al. Direct aerosol chemical composition measurements to evaluate the physicochemical differences between controlled sea spray aerosol generation schemes. Atmos. Meas. Tech. 7, 3667–3683 (2014).
Li, M. et al. Relative importance of gas uptake on aerosol and ground surfaces characterized by equivalent uptake coefficients. Atmos. Chem. Phys. 19, 10981–11011 (2019).
Li, S. et al. Contribution of vehicle emission and NO2 surface conversion to nitrous acid (HONO) in urban environments: implications from tests in a tunnel. Environ. Sci. Technol. 55, 15616–15624 (2021).
Yang, C. et al. New insights on the formation of nucleation mode particles in a coastal city based on a machine learning approach. Environ. Sci. Technol. 58, 1187–1198 (2024).
Hersbach, H. et al. The ERA5 global reanalysis. Q. J. R. Meteorol. Soc. 146, 1999–2049 (2020).
Fountoukis, C. & Nenes, A. Isorropia II: a computationally efficient thermodynamic equilibrium model for K+; Ca2+; Mg2+; NH4+; Na+; SO42-; NO3-; Cl-; H2O aerosols. Atmos. Chem. Phys. 7, 4639–4659 (2007).
Wolfe, G. M., Marvin, M. R., Roberts, S. J., Travis, K. R. & Liao, J. The framework for 0-D atmospheric modeling (F0AM) v3.1. Geosci. Model Dev. 9, 3309–3319 (2016).
Yan, C. et al. Increasing contribution of nighttime nitrogen chemistry to wintertime haze formation in Beijing observed during COVID-19 lockdowns. Nat. Geosci. 16, 975–981 (2023).
Lin, Z. et al. Trends of peroxyacetyl nitrate and its impact on ozone over 2018–2022 in urban atmosphere. npj Clim. Atmos. Sci. 7, 192 (2024).
Seinfeld, J. H., Pandis, S. N. & Noone, K. J. Atmospheric chemistry and physics: from air pollution to climate change. Phys. Today 51, 88–90 (1998).
Jacob, D. J. Chemistry of OH in remote clouds and its role in the production of formic acid and peroxymonosulfate. J. Geophys. Res. Atmos. 91, 9807–9826 (1986).
Gao, J. et al. Hydrogen peroxide serves as pivotal fountainhead for aerosol aqueous sulfate formation from a global perspective. Nat. Commun. 15, 4625 (2024).
Boyce, S. D. & Hoffmann, M. R. Kinetics and mechanism of the formation of hydroxymethanesulfonic acid at low pH. J. Phys. Chem. 88, 4740–4746 (1984).
Yu, C., Liu, T., Chi, X., Huang, X. & Ding, A. Ionic strength inhibits the multiphase reaction rate between dissolved SO2 and HCHO in aqueous aerosols: implications for sources of atmospheric hydroxymethanesulfonate. Environ. Sci. Technol. 59, 15900–15906 (2025).
Dovrou, E. et al. Catalytic role of formaldehyde in particulate matter formation. Proc. Natl. Acad. Sci. USA 119, e2113265119 (2022).
Acknowledgements
This work was funded by the National Natural Science Foundation of China (U22A20578), the guiding project of seizing the commanding heights of “self-purifying city” (IUE-CERAE-202402), the National Key Research and Development Program (2022YFC3700304), STS Plan Supporting Project of the Chinese Academy of Sciences in Fujian Province (2023T3013), and Xiamen Atmospheric Environment Observation and Research Station of Fujian Province.
Author information
Authors and Affiliations
Contributions
Z.L. contributed to the methodology, data curation, software, analysis and writing of the original draft. L.X. and J.C. contributed to the conceptualization, investigation, data curation, reviewing and editing the text, supervision, and funding acquisition. X.T., G.C., C.Y., K.Z., F.Z., L.L., and Y.C. provided useful advice and revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lin, Z., Ji, X., Xu, L. et al. Enhanced NO2-driven multiphase formation of particulate nitrate and sulfate under high-humidity conditions. npj Clim Atmos Sci (2026). https://doi.org/10.1038/s41612-026-01352-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41612-026-01352-5