Abstract
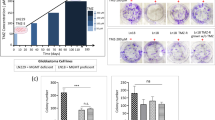
Glioma therapy often fails due to the acquisition of temozolomide (TMZ) resistance. Antiepileptic drugs, valproic acid (VPA) and levetiracetam (LEV) are commonly used during the perioperative period of glioma patients, and have been proposed for repurposing to augment TMZ efficacy. Our previous studies revealed that LEV could increase TMZ efficacy through downregulation of O-6-methylguanine-DNA methyltransferase (MGMT) and VPA could promote glioma cells apoptosis. Recently, tumor-associated macrophages (TAMs) have been considered as important modulators of TMZ resistance. As for LEV or VPA, which one is better to improve TMZ efficacy for macrophage-rich gliomas remains unclear. In this study, we investigated whether VPA or LEV could mitigate macrophage-mediated exceptional TMZ resistance. Our in vitro experiments revealed that VPA rather than LEV could polarize TAMs into M1 phenotypes. In vivo, glioma mouse models could benefit more from TMZ + VPA regimen by increasing intratumoral M1 macrophage infiltration. We conclude that VPA might be more useful than LEV to improve the therapeutic effect of TMZ for macrophage-rich gliomas. This study provides critical preclinical evidence for the optimal selection of the antiepileptic drug to overcome TAM-mediated chemoresistance, offering translational implications for personalized therapeutic strategies for macrophage-rich gliomas.
Similar content being viewed by others
Data availability
The key raw data was uploaded onto the Research Data Deposit public platform (www.researchdata.org.cn) with the RDD number as RDDB2025214027.
References
Price, M. et al. CBTRUS Statistical Report: primary brain and other central nervous system tumors diagnosed in the United States in 2017-2021. Neuro Oncol. 26, vi1–vi85 (2024).
Jiapaer, S., Furuta, T., Tanaka, S., Kitabayashi, T. & Nakada, M. Potential strategies overcoming the temozolomide resistance for glioblastoma. Neurol. Med. Chir. 58, 405–421 (2018).
Neftel, C. et al. An integrative model of cellular states, plasticity, and genetics for glioblastoma. Cell 178, 835–849 e821 (2019).
Wang, Q. et al. Tumor evolution of glioma-intrinsic gene expression subtypes associates with immunological changes in the microenvironment. Cancer Cell 32, 42–56 e46 (2017).
Li J. et al. PI3Kgamma inhibition suppresses microglia/TAM accumulation in glioblastoma microenvironment to promote exceptional temozolomide response. Proc. Natl. Acad. Sci. USA 118, e2009290118 (2021),
Nakada, M., Furuta, T., Hayashi, Y., Minamoto, T. & Hamada, J. The strategy for enhancing temozolomide against malignant glioma. Front. Oncol. 2, 98 (2012).
Ni, X. R. et al. Combination of levetiracetam and IFN-alpha increased temozolomide efficacy in MGMT-positive glioma. Cancer Chemother. Pharm. 86, 773–782 (2020).
Scicchitano, B. M. et al. Levetiracetam enhances the temozolomide effect on glioblastoma stem cell proliferation and apoptosis. Cancer Cell Int. 18, 136 (2018).
Fu, J., Shao, C. J., Chen, F. R., Ng, H. K. & Chen, Z. P. Autophagy induced by valproic acid is associated with oxidative stress in glioma cell lines. Neuro. Oncol. 12, 328–340 (2010).
Roos, W. P. et al. Intrinsic anticancer drug resistance of malignant melanoma cells is abrogated by IFN-beta and valproic acid. Cancer Res. 71, 4150–4160 (2011).
Basso, J., Miranda, A., Sousa, J., Pais, A. & Vitorino, C. Repurposing drugs for glioblastoma: from bench to bedside. Cancer Lett. 428, 173–183 (2018).
Hegi, M. E. et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N. Engl. J. Med. 352, 997–1003 (2005).
Li, Z. et al. Glioblastoma cell-derived lncRNA-containing exosomes induce microglia to produce complement C5, promoting chemotherapy resistance. Cancer Immunol. Res. 9, 1383–1399 (2021).
Ni, X. et al. Interrogating glioma-M2 macrophage interactions identifies Gal-9/Tim-3 as a viable target against PTEN-null glioblastoma. Sci. Adv. 8, eabl5165 (2022).
Chen, P. et al. Symbiotic macrophage-glioma cell interactions reveal synthetic lethality in PTEN-null glioma. Cancer Cell 35, 868–884 e866 (2019).
Lee, G. A. et al. IL-19 as a promising theranostic target to reprogram the glioblastoma immunosuppressive microenvironment. J. Biomed. Sci. 32, 34 (2025).
Cai, Z. et al. Valproic acid-like compounds enhance and prolong the radiotherapy effect on breast cancer by activating and maintaining anti-tumor immune function. Front. Immunol. 12, 646384 (2021).
Yang, Z. Y. & Wang, X. H. Valproic acid inhibits glioma and its mechanisms. J. Health. Eng. 2022, 4985781 (2022).
Verhaak, R. G. et al. Cancer genome atlas research n. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 17, 98–110 (2010).
Jia, D. et al. Mining TCGA database for genes of prognostic value in glioblastoma microenvironment. Aging 10, 592–605 (2018).
Wang, L. et al. A single-cell atlas of glioblastoma evolution under therapy reveals cell-intrinsic and cell-extrinsic therapeutic targets. Nat. Cancer 3, 1534–1552 (2022).
Chen, Z. & Hambardzumyan, D. Immune microenvironment in glioblastoma subtypes. Front. Immunol. 9, 1004 (2018).
Hambardzumyan, D., Gutmann, D. H. & Kettenmann, H. The role of microglia and macrophages in glioma maintenance and progression. Nat. Neurosci. 19, 20–27 (2016).
Pombo Antunes, A. R. et al. Single-cell profiling of myeloid cells in glioblastoma across species and disease stage reveals macrophage competition and specialization. Nat. Neurosci. 24, 595–610 (2021).
Froscher, W., Kirschstein, T. & Rosche, J. Anticonvulsant therapy for brain tumour-related epilepsy. Fortschr. Neurol. Psychiatr. 82, 678–690 (2014).
Kerkhof, M. et al. Effect of valproic acid on seizure control and on survival in patients with glioblastoma multiforme. Neuro. Oncol. 15, 961–967 (2013).
Bobustuc, G. C. et al. Levetiracetam enhances p53-mediated MGMT inhibition and sensitizes glioblastoma cells to temozolomide. Neuro. Oncol. 12, 917–927 (2010).
Mohammadi, S., Saghaeian-Jazi, M., Sedighi, S. & Memarian, A. Sodium valproate modulates immune response by alternative activation of monocyte-derived macrophages in systemic lupus erythematosus. Clin. Rheumatol. 37, 719–727 (2018).
Shen, D. et al. Interferon-alpha/beta enhances temozolomide activity against MGMT-positive glioma stem-like cells. Oncol. Rep. 34, 2715–2721 (2015).
Natsume, A. et al. A combination of IFN-beta and temozolomide in human glioma xenograft models: implication of p53-mediated MGMT downregulation. Cancer Chemother. Pharm. 61, 653–659 (2008).
Chen, P. et al. Gpr132 sensing of lactate mediates tumor-macrophage interplay to promote breast cancer metastasis. Proc. Natl. Acad. Sci. USA 114, 580–585 (2017).
Ni, X. R. et al. Transferrin receptor 1 targeted optical imaging for identifying glioma margin in mouse models. J. Neurooncol. 148, 245–258 (2020).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587 e3529 (2021).
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (NSFC) (81872059), Cancer Innovative Research Program of Sun Yat-sen University Cancer Center (CIRP-SYSUCC-PT13120101), the Natural Science Foundation of Guangdong Province (NSFG) (2025A1515012554), the China Postdoctoral Science Foundation (2022M721513 and 2024T170384) and the President Foundation of Zhujiang Hospital, Southern Medical University (yzjj2022qn06).
Author information
Authors and Affiliations
Contributions
X.R.N. and Z.P.C. conceived the ideas and wrote the primary manuscript. X.R.N. performed and analyzed most of experiments and produced figures. X.R.N., J.Z., X.D.Q., and Z.P.C. wrote, edited, and reviewed the manuscript. PFX completed the bioinformatics analysis of the scRNA-seq and bulk RNA-seq data and produced the related figures. W.B.C., F.R.C., and X.R.N. completed the animal experiments. M.H.C., Y.L.Y., and C.C.G. completed the experiments of in vitro glioma explants treatment and the IHC analysis. All authors read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ni, X., Chen, W., Xu, P. et al. Valproic acid reverses macrophage-mediated temozolomide resistance in macrophage-rich gliomas. npj Precis. Onc. (2026). https://doi.org/10.1038/s41698-026-01325-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41698-026-01325-0