Fig. 1: A strategic approach to incorporating a digital health technology and digitally derived endpoint within a clinical development program.
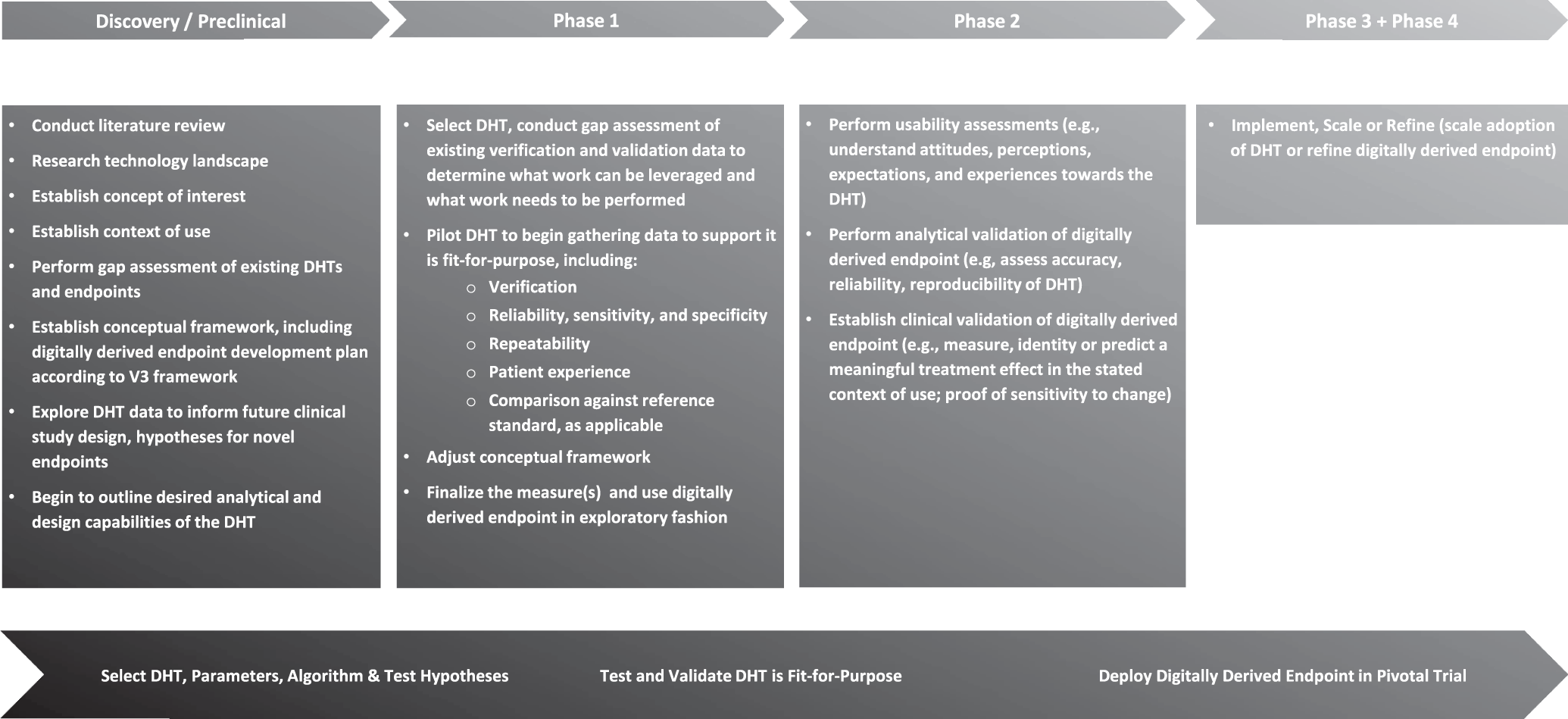
To demonstrate that the digital health technology (DHT) is fit-for-purpose in regulatory decision making, all of the steps here should be completed, but the order of execution and when they are completed, in terms of drug development phases, may vary. Certain drug development programs may not follow the precise three-phase structure shown here, and some activities shown here may be completed in parallel with one another. In a regulatory context, the concept of interest is the aspect of an individual’s clinical, biological, physical, or functional state, or experience that the assessment is intended to capture (or reflect). Context of Use is a statement that fully and clearly describes the way the medical product development tool is to be used and the regulated product development and review-related purpose of the use. V3 framework2 refers to a three-stage process of Verifying, analytically Validating, and clinically Validating a Biometric Monitoring Tool (BioMeT) to demonstrate it is fit-for-purpose for gathering data in a clinical trial.
