Abstract
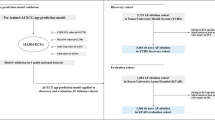
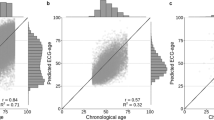
Artificial intelligence (AI)-derived electrocardiographic (ECG) age is a promising marker of atrial fibrillation (AF) risk. We developed PROPHECG-Age Single—an AI model estimating ECG age from wearable single-lead ECGs—and examined whether the ECG-age gap (predicted minus chronological age) is associated with AF presence and burden in real-world self-monitoring context. One million 12-lead ECGs from a hospital were converted to synthetic single-lead signals via Cycle-Consistent Generative Adversarial Network and used to train a residual network-based model. Validation in two independent wearable cohorts (S-Patch [ClinicalTrials.gov: NCT05119725, registered November 2021]; Memo Patch [ClinicalTrials.gov: NCT05355948, registered May 2022]) showed mean absolute errors of 10.01 and 11.88 years, respectively. The pooled association with AF presence was significant (odds ratio 1.03 per 1-year gap), and for AF burden, each 1-year gap increase corresponded to a 0.8 percentage point rise. These findings support wearable-based AI-ECG age as a potential digital biomarker for proactive cardiovascular monitoring.
Similar content being viewed by others
Data availability
The anonymised ECG datasets used in this study are not publicly available due to patient privacy restrictions and institutional data protection policies. However, the data will be made available to qualified investigators for the purpose of replicating the analyses and findings upon reasonable request to the corresponding authors, subject to appropriate ethical approvals and institutional authorizations.
Code availability
The complete AI algorithm (PROPHECG-Age Single), including source code and pre-trained model weights, is openly available on GitHub (https://github.com/dr-you-group/PROPHECG-Age-Single) and archived on Zenodo with the identifier https://doi.org/10.5281/zenodo.18218561. Any additional custom scripts used for data preprocessing are available from the corresponding authors upon reasonable request.
References
Roberts, J. D. et al. Epigenetic age and the risk of incident atrial fibrillation. Circulation 144, 1899–1911 (2021).
Hamczyk, M. R., Nevado, R. M., Barettino, A., Fuster, V. & Andrés, V. Biological versus chronological aging: JACC focus seminar. J. Am. Coll. Cardiol. 75, 919–930 (2020).
Linz, D. et al. Atrial fibrillation: epidemiology, screening and digital health. Lancet Reg. Health Eur. 37, 100786 (2024).
Freedman, B. et al. World Heart Federation roadmap on atrial fibrillation—a 2020 update. Glob. Heart 16, 41 (2021).
Lima, E. M. et al. Deep neural network-estimated electrocardiographic age as a mortality predictor. Nat. Commun. 12, 5117 (2021).
Saleh, G. et al. Artificial intelligence electrocardiogram-derived heart age predicts long-term mortality after transcatheter aortic valve replacement. JACC Adv. 3, 101171 (2024).
Cho, S. et al. Artificial intelligence–derived electrocardiographic aging and risk of atrial fibrillation: a multi-national study. Eur. Heart J. 46, 839–852 (2025).
Park, H. et al. Artificial intelligence estimated electrocardiographic age as a recurrence predictor after atrial fibrillation catheter ablation. npj Digit. Med. 7, 234 (2024).
Attia, Z. I. et al. Age and sex estimation using artificial intelligence from standard 12-lead ECGs. Circ. Arrhythm. Electrophysiol. 12, e007284 (2019).
Mossavarali, S. et al. Determinants of artificial intelligence electrocardiogram-derived age and its association with cardiovascular events and mortality: a systematic review and meta-analysis. npj Digit. Med. 8, 1–13 (2025).
Gundlapalle, V. & Acharyya, A. Proc. IEEE 13th Latin America Symposium on Circuits and System (LASCAS) 01–04 (IEEE, 2022).
Seo, H.-C., Yoon, G.-W., Joo, S. & Nam, G.-B. Multiple electrocardiogram generator with single-lead electrocardiogram. Comput. Methods Programs Biomed. 221, 106858 (2022).
Obianom, E. N., Ng, G. A. & Li, X. Reconstruction of 12-lead ECG: a review of algorithms. Front. Physiol. 16, 1532284 (2025).
Presacan, O. et al. Evaluating the feasibility of 12-lead electrocardiogram reconstruction from limited leads using deep learning. Commun. Med. 5, 139 (2025).
Shin, S. J. et al. Style transfer strategy for developing a generalizable deep learning application in digital pathology. Comput. Methods Programs Biomed. 198, 105815 (2021).
Attia, Z. I. et al. An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: a retrospective analysis of outcome prediction. Lancet 394, 861–867 (2019).
Rivner, H., Mitrani, R. D. & Goldberger, J. J. Atrial myopathy underlying atrial fibrillation. Arrhythm. Electrophysiol. Rev. 9, 61 (2020).
Charitos, E. I., Pürerfellner, H., Glotzer, T. V. & Ziegler, P. D. Clinical classifications of atrial fibrillation poorly reflect its temporal persistence: insights from 1,195 patients continuously monitored with implantable devices. J. Am. Coll. Cardiol. 63, 2840–2848 (2014).
Schlesinger, D. E. et al. Artificial intelligence for hemodynamic monitoring with a wearable electrocardiogram monitor. Commun. Med. 5, 4 (2025).
Mohebbian, M. R. et al. Fetal ECG extraction from maternal ECG using attention-based CycleGAN. IEEE J. Biomed. Health Inform. 26, 515–526 (2021).
Alonso, A. et al. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF consortium. J. Am. Heart Assoc. 2, e000102 (2013).
Acknowledgements
This study was funded by the Ministry of Health & Welfare, Republic of Korea (RS-2022-KH125397, RS-2022-KH129902, RS-2023-00265440, RS-2024-00397290, HI22C0452), the Ministry of Science and ICT, Republic of Korea (RS-2025-24533659), Samjin Pharmaceutical, Yuhan Corporation, Wellysis, and HUINNO. We express our gratitude to Severance Hospital for providing invaluable ECG data that made this research possible. S.H.P. acknowledges support from the Yonsei University College of Medicine MSTP Scholarship. We also appreciate Daeun Joung for her support.
Author information
Authors and Affiliations
Contributions
S.H.P. led the study, taking primary responsibility for manuscript drafting (including tables and figures), AI model development, and data analysis. J.H.J. contributed to data analysis and provided research assistance. B.J. designed and supervised the cohort studies. S.C.Y. contributed to model development, designed the statistical analysis plan, and provided overall supervision. The study concept was developed jointly by S.C.Y., H.T.Y., and B.J., who also offered critical feedback throughout the project. J.K., D.L., D.K., and J.J. (Industry collaborators) secured, processed, and provided the single-lead wearable ECG data. All authors reviewed and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
S.H.P. declares no competing interests beyond the institutional funding reported above. J.H.J., D.L., and H.T.Y. declare no competing interests. J.K. is a shareholder of Wellysis Corp. and reports pending patent applications related to atrial fibrillation prediction using AI (United States Application No. 18/636,402, filed 15 April 2024; Republic of Korea Application No. 10-2023-0069397, filed 30 May 2023). D.K. and J.J. are shareholders of HUINNO Corp. S.C.Y. serves as the Chief Executive Officer of PHI Digital Healthcare, reports grants from Daiichi Sankyo, and is a coinventor of granted Korean Patents (DP-2023-1223, DP-2023-0920) and pending Patent Applications (DP-2024-0909, DP-2024-0908, DP-2022-1658, DP-2022-1478, DP-2022-1365, PATENT-2025-0039190, PATENT-2025-0039191, PATENT-2025-0039192, PATENT-2025-0039193, PATENT-2025-0039194), all unrelated to the present work. B.J. has served as a speaker for Bayer, BMS/Pfizer, Medtronic, and Daiichi-Sankyo, and received research funds from Samjin, Yuhan, Medtronic, Boston Scientific, and Abbott Korea.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Park, S.H., Jin, J.H., Kim, J. et al. Wearable device derived electrocardiographic age and its association with atrial fibrillation. npj Digit. Med. (2026). https://doi.org/10.1038/s41746-026-02344-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41746-026-02344-8