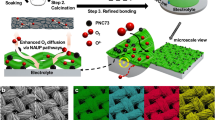
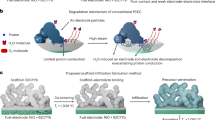
Abstract
Intermediate-temperature protonic ceramic electrolysis cells (PCECs), which combine the benefits of both lower- and higher-temperature electrolysis, are among the most efficient technologies for the production of green hydrogen. To ensure economic competitiveness and broad adoption, ongoing innovations in cell materials are essential to improve durability and reduce costs. The water oxidation half-reaction at the anode is a key area for improvement as it is a major contributor to performance degradation and efficiency loss in PCECs. Current anode designs, which are largely derived from solid oxide electrolysis cells, fail to address the specific requirements for PCECs under realistic operating conditions. This Perspective highlights the unique challenges faced by PCEC anodes, focusing on the impact of high steam concentrations and the critical role of proton-coupled electron-transfer mechanisms—factors that are absent in solid oxide electrolysis cells. Furthermore, we explore design principles for advancing anodes tailored for PCECs, offering guidance for future research and development in this promising field.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lagadec, M. F. & Grimaud, A. Water electrolysers with closed and open electrochemical systems. Nat. Mater. 19, 1140–1150 (2020).
Zhang, W. et al. Water electrolysis toward elevated temperature: advances, challenges and frontiers. Chem. Rev. 123, 7119–7192 (2023).
Hauch, A. et al. Recent advances in solid oxide cell technology for electrolysis. Science 370, eaba6118 (2020).
Liu, F. et al. Lowering the operating temperature of protonic ceramic electrochemical cells to <450 °C. Nat. Energy 8, 1145–1157 (2023).
Choi, S. et al. Exceptional power density and stability at intermediate temperatures in protonic ceramic fuel cells. Nat. Energy 3, 202–210 (2018).
Yu, H. et al. Lowering the temperature of solid oxide electrochemical cells using triple-doped bismuth oxides. Adv. Mater. 36, 2306205 (2024).
Duan, C., Huang, J., Sullivan, N. & O’Hayre, R. Proton-conducting oxides for energy conversion and storage. Appl. Phys. Rev. 7, 011314 (2020).
Zhai, S. et al. A combined ionic Lewis acid descriptor and machine-learning approach to prediction of efficient oxygen reduction electrodes for ceramic fuel cells. Nat. Energy 7, 866–875 (2022).
Wang, Z. et al. Rational design of perovskite ferrites as high-performance proton-conducting fuel cell cathodes. Nat. Catal. 5, 777–787 (2022).
Choi, M. et al. Exceptionally high performance of protonic ceramic fuel cells with stoichiometric electrolytes. Energy Environ. Sci. 14, 6476–6483 (2021).
An, H. et al. An unprecedented vapor-phase sintering activator for highly refractory proton-conducting oxides. ACS Energy Lett. 7, 4036–4044 (2022).
Liu, F. & Duan, C. in High-Temperature Electrolysis: From Fundamentals to Applications (eds Sitte, W. & Merkle, R.) Ch. 14 (IOP Publishing, 2023); https://doi.org/10.1088/978-0-7503-3951-3ch14
Tao, H. B. et al. The gap between academic research on proton exchange membrane water electrolysers and industrial demands. Nat. Nanotechnol. 19, 1074–1076 (2024).
Subotić, V. & Hochenauer, C. Analysis of solid oxide fuel and electrolysis cells operated in a real-system environment: State-of-the-health diagnostic, failure modes, degradation mitigation and performance regeneration. Prog. Energy Combust. Sci. 93, 101011 (2022).
Wang, Y. et al. A review of progress in proton ceramic electrochemical cells: material and structural design, coupled with value-added chemical production. Energy Environ. Sci. 16, 5721–5770 (2023).
Wachsman, E. D. & Lee, K. T. Lowering the temperature of solid oxide fuel cells. Science 334, 935–939 (2011).
Liu, F., Ding, D. & Duan, C. Protonic ceramic electrochemical cells for synthesizing sustainable chemicals and fuels. Adv. Sci. 10, 2206478 (2023).
Li, M. et al. Switching of metal–oxygen hybridization for selective CO2 electrohydrogenation under mild temperature and pressure. Nat. Catal. 4, 274–283 (2021).
Li, M. et al. Activating nano-bulk interplays for sustainable ammonia electrosynthesis. Mater. Today 60, 31–40 (2022).
Clark, D. et al. Single-step hydrogen production from NH3, CH4, and biogas in stacked proton ceramic reactors. Science 376, 390–393 (2022).
Pei, K. et al. Surface restructuring of a perovskite-type air electrode for reversible protonic ceramic electrochemical cells. Nat. Commun. 13, 2207 (2022).
Wu, W. et al. 3D self-architectured steam electrode enabled efficient and durable hydrogen production in a proton-conducting solid oxide electrolysis cell at temperatures lower than 600 °C. Adv. Sci. 5, 1800360 (2018).
Song, Y., Yi, Y., Ran, R., Zhou, W. & Wang, W. Recent advances in barium cobaltite-based perovskite oxides as cathodes for intermediate-temperature solid oxide fuel cells. Small 20, 2406627 (2024).
Zou, D. et al. The BaCe0.16Y0.04Fe0.8O3−δ nanocomposite: a new high-performance cobalt-free triple-conducting cathode for protonic ceramic fuel cells operating at reduced temperatures. J. Mater. Chem. A 10, 5381–5390 (2022).
Zhang, W. et al. A synergistic three-phase, triple-conducting air electrode for reversible proton-conducting solid oxide cells. ACS Energy Lett. 8, 3999–4007 (2023).
He, F. et al. Phase segregation of a composite air electrode unlocks the high performance of reversible protonic ceramic electrochemical cells. Energy Environ. Sci. 17, 3898–3907 (2024).
Chen, X. et al. Synergistic bulk and surface engineering for expeditious and durable reversible protonic ceramic electrochemical cells air electrode. Adv. Mater. 36, 2403998 (2024).
Regalado Vera, C. Y. et al. Improving proton conductivity by navigating proton trapping in high scandium-doped barium zirconate electrolytes. Chem. Mater. 35, 5341–5352 (2023).
Rowberg, A. J. E., Li, M., Ogitsu, T. & Varley, J. B. Incorporation of protons and hydroxide species in BaZrO3 and BaCeO3. Mater. Adv. 4, 6233–6243 (2023).
Rowberg, A. J. E., Li, M., Ogitsu, T. & Varley, J. B. Polarons and electrical leakage in BaZrO3 and BaCeO3. Phys. Rev. Mater. 7, 015402 (2023).
Jang, I. et al. Electrocatalysis in solid oxide fuel cells and electrolyzers. Chem. Rev. 124, 8233–8306 (2024).
Li, M., Hua, B., Chen, J., Zhong, Y. & Luo, J.-L. Charge transfer dynamics in RuO2/perovskite nanohybrid for enhanced electrocatalysis in solid oxide electrolyzers. Nano Energy 57, 186–194 (2019).
Koo, B. et al. Sr segregation in perovskite oxides: why it happens and how it exists. Joule 2, 1476–1499 (2018).
Norby, T. Protonic conduction on oxide surfaces—role and applications in electrochemical energy conversion. Prog. Energy 6, 043002 (2024).
Su, H. & Hu, Y. H. Degradation issues and stabilization strategies of protonic ceramic electrolysis cells for steam electrolysis. Energy Sci. Eng. 10, 1706–1725 (2022).
Pan, J., Ye, Y., Zhou, M., Sun, X. & Chen, Y. Revealing the impact of steam concentration on the activity and stability of double-perovskite air electrodes for proton-conducting electrolysis cells. Energy Fuels 36, 12253–12260 (2022).
Sengodan, S. et al. Layered oxygen-deficient double perovskite as an efficient and stable anode for direct hydrocarbon solid oxide fuel cells. Nat. Mater. 14, 205–209 (2015).
Huan, D. et al. New, efficient, and reliable air electrode material for proton-conducting reversible solid oxide cells. ACS Appl. Mater. Interfaces 10, 1761–1770 (2018).
Tang, W. et al. An unbalanced battle in excellence: revealing effect of Ni/Co occupancy on water splitting and oxygen reduction reactions in triple-conducting oxides for protonic ceramic electrochemical cells. Small 18, 2201953 (2022).
Koper, M. T. M. Theory of multiple proton–electron transfer reactions and its implications for electrocatalysis. Chem. Sci. 4, 2710 (2013).
Tyburski, R., Liu, T., Glover, S. D. & Hammarström, L. Proton-coupled electron transfer guidelines, fair and square. J. Am. Chem. Soc. 143, 560–576 (2021).
Li, J. Oxygen evolution reaction in energy conversion and storage: eesign strategies under and beyond the energy scaling relationship. Nanomicro Lett. 14, 112 (2022).
Hwang, J. et al. Perovskites in catalysis and electrocatalysis. Science 358, 751–756 (2017).
Huang, J. et al. Modifying redox properties and local bonding of Co3O4 by CeO2 enhances oxygen evolution catalysis in acid. Nat. Commun. 12, 3036 (2021).
Zhu, Y. et al. Tuning proton-coupled electron transfer by crystal orientation for efficient water oxidization on double perovskite oxides. Nat. Commun. 11, 4299 (2020).
Ding, H. et al. Self-sustainable protonic ceramic electrochemical cells using a triple conducting electrode for hydrogen and power production. Nat. Commun. 11, 1907 (2020).
Bian, W. et al. Revitalizing interface in protonic ceramic cells by acid etch. Nature 604, 479–485 (2022).
Zhang, Y., Chen, Y., Yan, M. & Chen, F. Reconstruction of relaxation time distribution from linear electrochemical impedance spectroscopy. J. Power Sources 283, 464–477 (2015).
Choi, M. et al. Interface engineering to operate reversible protonic ceramic electrochemical cells below 500 °C. Adv. Energy Mater. 15, 2400124 (2024).
Osipov, A. A. High-temperature Raman spectroscopy. Pure Appl. Chem. 91, 1749–1756 (2019).
Hartman, T., Geitenbeek, R. G., Whiting, G. T. & Weckhuysen, B. M. Operando monitoring of temperature and active species at the single catalyst particle level. Nat. Catal. 2, 986–996 (2019).
Esterhuizen, J. A., Goldsmith, B. R. & Linic, S. Interpretable machine learning for knowledge generation in heterogeneous catalysis. Nat. Catal. 5, 175–184 (2022).
Resasco, J. et al. Enhancing the connection between computation and experiments in electrocatalysis. Nat. Catal. 5, 374–381 (2022).
An, H. et al. A 5 × 5 cm2 protonic ceramic fuel cell with a power density of 1.3 W cm−2 at 600 °C. Nat. Energy 3, 870–875 (2018).
Song, Y. et al. Self-assembled triple-conducting nanocomposite as a superior protonic ceramic fuel cell cathode. Joule 3, 2842–2853 (2019).
Bae, K. et al. Demonstrating the potential of yttrium-doped barium zirconate electrolyte for high-performance fuel cells. Nat. Commun. 8, 14553 (2017).
Bae, K., Kim, D. H., Choi, H. J., Son, J.-W. & Shim, J. H. High-performance protonic ceramic fuel cells with 1 µm thick Y:Ba(Ce, Zr)O3 electrolytes. Adv. Energy Mater. 8, 1801315 (2018).
Acknowledgements
This work is supported by the proton-conducting solid oxide electrolysis cells (P-SOEC) lab call project and the HydroGEN Advanced Water Splitting Materials Consortium, established as part of the Energy Materials Network under the US Department of Energy (USDOE); the Office of Energy Efficiency and Renewable Energy (EERE); the Hydrogen and Fuel Cell Technologies Office (HFTO) under the USDOE Idaho Operations Office under contract no. DE-AC07-05ID14517. M.L. is grateful for the support from the Idaho National Laboratory’s Laboratory Directed Research and Development program (project no. 23A1070-027FP) under the USDOE Idaho Operations Office under contract no. DE-AC07-05ID14517.
Author information
Authors and Affiliations
Contributions
D.D. conceived the idea of this Perspective. M.L. and F.L. wrote the first draught of the paper. All authors contributed to reviewing and editing the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Wei Wang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, M., Liu, F. & Ding, D. Critical insights into the steam electrolysis electrode in protonic ceramic cells for hydrogen production. Nat Catal 8, 293–300 (2025). https://doi.org/10.1038/s41929-025-01313-w
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41929-025-01313-w