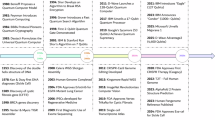
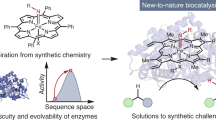
Abstract
Quantum computing, by leveraging the unique principles of quantum mechanics, offers transformative potential for biocatalysis and related disciplines. Compared to classical algorithms, quantum algorithms deliver immense acceleration to quantum computers, making them suited for tackling computationally challenging problems such as simulating many-body biomolecular systems or enzyme-catalysed chemical reactions. However, current quantum hardware is constrained by noise, limited qubit coherence and high error rates, restricting its capacity to model complex biochemical phenomena. Here we explore the rapidly advancing landscape of quantum computing and its future applications in the discovery and rational engineering of biocatalysts. We identify key areas where quantum algorithms could surpass classical limitations, including the quantum chemistry-based design of biocatalysts with enhanced catalytic activity or selectivity, parallelized mining of novel enzymes, accurate ancestral sequence reconstruction, and combinatorial in silico protein evolution. Overcoming current hardware limitations could unlock transformative advances in both fundamental enzymology and industrial bioprocessing.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Nielsen, M. A. & Chuang, I. L. Quantum Computation and Quantum Information: 10th Anniversary Edition (Cambridge Univ. Press, 2010).
Chandran, A. Biopharma foresees a ‘quantum advantage’: they could be right. Nat. Biotechnol. 42, 690–692 (2024).
Kissman, E. N. et al. Expanding chemistry through in vitro and in vivo biocatalysis. Nature 631, 37–48 (2024).
Buller, R. et al. From nature to industry: harnessing enzymes for biocatalysis. Science 382, eadh8615 (2023).
Schumacher, B. Quantum coding. Phys. Rev. A 51, 2738–2747 (1995).
Besedin, I. et al. Realizing lattice surgery on two distance–three repetition codes with superconducting qubits. Preprint at https://doi.org/10.48550/arXiv.2501.04612 (2025).
Matsunaga, H. & Ho, L. B. Detecting and protecting entanglement through nonlocality, variational entanglement witness and nonlocal measurements. Phys. Rev. Res. 7, 013239 (2025).
Kitaev, A. Y. Quantum measurements and the Abelian Stabilizer Problem. Preprint at https://doi.org/10.48550/arXiv.quant-ph/9511026 (1995).
Acharya, R. et al. Suppressing quantum errors by scaling a surface code logical qubit. Nature 614, 676–681 (2023).
Bogobowicz, M. et al. Quantum Technology Sees Record Investments, Progress on Talent Gap (McKinsey, 2023); https://www.mckinsey.com/capabilities/mckinsey-digital/our-insights/quantum-technology-sees-record-investments-progress-on-talent-gap
Shor, P. W. Polynomial-time algorithms for prime factorization and discrete logarithms on a quantum computer. SIAM J. Comput. 26, 1484–1509 (1997).
Feynman, R. P. Simulating physics with computers. Int. J. Theor. Phys. 21, 467–488 (1982).
Babbush, R. et al. Focus beyond quadratic speedups for error-corrected quantum advantage. PRX Quantum 2, 010103 (2021).
Aaronson, S. Read the fine print. Nat. Phys. 11, 291–293 (2015).
Arute, F. et al. Quantum supremacy using a programmable superconducting processor. Nature 574, 505–510 (2019).
Zhao, X.-H. et al. Leapfrogging Sycamore: harnessing 1432 GPUs for 7× faster quantum random circuit sampling. Natl Sci. Rev. 12, nwae317 (2025).
Ball, P. Physicists in China challenge Google’s ‘quantum advantage’. Nature 588, 380 (2020).
Acharya, R. et al. Quantum error correction below the surface code threshold. Nature 638, 920–926 (2025).
King, A. D. et al. Beyond-classical computation in quantum simulation. Science 388, 199–204 (2025).
Pal, S., Bhattacharya, M., Lee, S.-S. & Chakraborty, C. Quantum computing in the next-generation computational biology landscape: from protein folding to molecular dynamics. Mol. Biotechnol. 66, 163–178 (2024).
Doga, H. et al. A perspective on protein structure prediction using quantum computers. J. Chem. Theory Comput. 20, 3359–3378 (2024).
Nałęcz-Charkiewicz, K., Charkiewicz, K. & Nowak, R. M. Quantum computing in bioinformatics: a systematic review mapping. Brief. Bioinform. 25, bbae391 (2024).
Liu, H. et al. Prospects of quantum computing for molecular sciences. Mater. Theory 6, 11 (2022).
Peruzzo, A. et al. A variational eigenvalue solver on a photonic quantum processor. Nat. Commun. 5, 4213 (2014).
Grimsley, G. R. et al. Increasing protein stability by altering long-range coulombic interactions. Protein Sci. 8, 1843–1849 (1999).
Robert, A., Barkoutsos, P. K., Woerner, S. & Tavernelli, I. Resource-efficient quantum algorithm for protein folding. npj Quantum Inf. 7, 38 (2021).
Ettenhuber, P. et al. Calculating the energy profile of an enzymatic reaction on a quantum computer. J. Chem. Theory Comput. 21, 3493–3503 (2025).
Malone, F. D. et al. Towards the simulation of large scale protein–ligand interactions on NISQ-era quantum computers. Chem. Sci. 13, 3094–3108 (2022).
Aramyan, S., McGregor, K., Sandeep, S. & Haczku, A. SP-A binding to the SARS-CoV-2 spike protein using hybrid quantum and classical in silico modeling and molecular pruning by quantum approximate optimization algorithm (QAOA) based MaxCut with ZDOCK. Front. Immunol 13, 945317 (2022).
Pamidimukkala, J. V. et al. Protein structure prediction with high degrees of freedom in a gate-based quantum computer. J. Chem. Theory Comput. 20, 10223–10234 (2024).
Papalitsas, C. et al. Quantum approximate optimization algorithms for molecular docking. Preprint at https://doi.org/10.48550/arXiv.2503.04239 (2025).
Chagneau, A., Massaoudi, Y., Derbali, I. & Yahiaoui, L. Quantum algorithm for bioinformatics to compute the similarity between proteins. IET Quantum Commun. 5, 417–442 (2024).
Banchi, L., Fingerhuth, M., Babej, T., Ing, C. & Arrazola, J. M. Molecular docking with Gaussian boson sampling. Sci. Adv. 6, eaax1950 (2020).
Fox, D. M., Branson, K. M. & Walker, R. C. mRNA codon optimization with quantum computers. PLoS ONE 16, e0259101 (2021).
Khatami, M. H., Mendes, U. C., Wiebe, N. & Kim, P. M. Gate-based quantum computing for protein design. PLoS Comput. Biol. 19, e1011033 (2023).
Grover, L. K. A fast quantum mechanical algorithm for database search. In Proc. Twenty-Eighth Annual ACM Symposium on Theory of Computing (ed. Miller, G. L.) 212–219 (Association for Computing Machinery, 1996); https://doi.org/10.1145/237814.237866
Allcock, J. et al. The prospects of Monte Carlo antibody loop modelling on a fault-tolerant quantum computer. Front. Drug Discov 2, 908870 (2022).
McArdle, S., Endo, S., Aspuru-Guzik, A., Benjamin, S. C. & Yuan, X. Quantum computational chemistry. Rev. Mod. Phys. 92, 015003 (2020).
Ma, P., Chen, Y., Lu, H. & Zhong, W. Bisection Grover’s search algorithm and its application in analyzing CITE-seq data. J. Am. Stat. Assoc. 120, 52–63 (2024).
Kundu, D. et al. Application of quantum tensor networks for protein classification. In Proc. Great Lakes Symposium on VLSI 2024 (eds Partin-Vaisband, I. et al.) 132–137 (Association for Computing Machinery, 2024); https://doi.org/10.1145/3649476.3658701
Ghazi Vakili, M. et al. Quantum-computing-enhanced algorithm unveils potential KRAS inhibitors. Nat. Biotechnol. https://doi.org/10.1038/s41587-024-02526-3 (2025).
Kouba, P. et al. Machine learning-guided protein engineering. ACS Catal. 13, 13863–13895 (2023).
Jayakumar, A. et al. Quantum algorithm implementations for beginners. ACM Trans. Quantum Comput 3, 18:1–18:92 (2022).
Protein Engineering Portal (Loschmidt Laboratories); https://loschmidt.chemi.muni.cz/portal/
Marques, S. M. et al. Caver Web 2.0: analysis of tunnels and ligand transport in dynamic ensembles of proteins. Nucl. Acids Res. 53, W132–W142 (2025).
Hollingsworth, S. A. & Dror, R. O. Molecular dynamics simulation for all. Neuron 99, 1129–1143 (2018).
Gerlt, J. A. et al. Enzyme Function Initiative-Enzyme Similarity Tool (EFI-EST): a web tool for generating protein sequence similarity networks. Biochim. Biophys. Acta Proteins Proteom. 1854, 1019–1037 (2015).
Hon, J. et al. EnzymeMiner: automated mining of soluble enzymes with diverse structures, catalytic properties and stabilities. Nucl. Acids Res. 48, W104–W109 (2020).
Hon, J. et al. SoluProt: prediction of soluble protein expression in Escherichia coli. Bioinformatics 37, 23–28 (2021).
Planas-Iglesias, J. et al. AggreProt: a web server for predicting and engineering aggregation prone regions in proteins. Nucl. Acids Res. 52, W159–W169 (2024).
Vasina, M. et al. Advanced database mining of efficient haloalkane dehalogenases by sequence and structure bioinformatics and microfluidics. Chem. Catal. 2, 2704–2725 (2022).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Lloyd, S. Universal quantum simulators. Science 273, 1073–1078 (1996).
Daley, A. J. et al. Practical quantum advantage in quantum simulation. Nature 607, 667–676 (2022).
Babbush, R. et al. Quantum simulation of exact electron dynamics can be more efficient than classical mean-field methods. Nat. Commun. 14, 4058 (2023).
Musil, M. et al. FireProtASR: a web server for fully automated ancestral sequence reconstruction. Brief. Bioinform. 22, bbaa337 (2021).
Ross, C. M., Foley, G., Boden, M. & Gillam, E. M. J. in Enzyme Engineering: Methods and Protocols (eds Magnani, F. et al.) 85–110 (Springer, 2022); https://doi.org/10.1007/978-1-0716-1826-4_6
Onodera, W., Hara, N., Aoki, S., Asahi, T. & Sawamura, N. Phylogenetic tree reconstruction via graph cut presented using a quantum-inspired computer. Mol. Phylogenet. Evol. 178, 107636 (2023).
Watson, J. L. et al. De novo design of protein structure and function with RFdiffusion. Nature 620, 1089–1100 (2023).
McClean, J. R., Romero, J., Babbush, R. & Aspuru-Guzik, A. The theory of variational hybrid quantum-classical algorithms. N. J. Phys. 18, 023023 (2016).
Chovancova, E. et al. CAVER 3.0: a tool for the analysis of transport pathways in dynamic protein structures. PLoS Comput. Biol. 8, e1002708 (2012).
Vavra, O. et al. CaverDock: a molecular docking-based tool to analyse ligand transport through protein tunnels and channels. Bioinformatics 35, 4986–4993 (2019).
Horn, D. & Gottlieb, A. Algorithm for data clustering in pattern recognition problems based on quantum mechanics. Phys. Rev. Lett. 88, 018702 (2001).
Ollitrault, P. J., Miessen, A. & Tavernelli, I. Molecular quantum dynamics: a quantum computing perspective. Acc. Chem. Res. 54, 4229–4238 (2021).
Grimsley, H. R., Economou, S. E., Barnes, E. & Mayhall, N. J. An adaptive variational algorithm for exact molecular simulations on a quantum computer. Nat. Commun. 10, 3007 (2019).
Sheng, X., Kazemi, M., Planas, F. & Himo, F. Modeling enzymatic enantioselectivity using quantum chemical methodology. ACS Catal. 10, 6430–6449 (2020).
Goings, J. J. et al. Reliably assessing the electronic structure of cytochrome P450 on today’s classical computers and tomorrow’s quantum computers. Proc. Natl Acad. Sci. USA 119, e2203533119 (2022).
Nguyen, T. D., Chen, Y.-I., Chen, L. H. & Yeh, H.-C. Recent advances in single-molecule tracking and imaging techniques. Annu. Rev. Anal. Chem. 16, 253–284 (2023).
Vasina, M. et al. In-depth analysis of biocatalysts by microfluidics: an emerging source of data for machine learning. Biotechnol. Adv. 66, 108171 (2023).
Rapp, J. T., Bremer, B. J. & Romero, P. A. Self-driving laboratories to autonomously navigate the protein fitness landscape. Nat. Chem. Eng. 1, 97–107 (2024).
Cai, H. et al. Brain organoid reservoir computing for artificial intelligence. Nat. Electron. 6, 1032–1039 (2023).
Lu, C. et al. The AI Scientist: towards fully automated open-ended scientific discovery. Preprint at https://doi.org/10.48550/arXiv.2408.06292 (2024).
Google Quantum AI; https://quantumai.google/
Quantum Roadmap; https://www.ibm.com/roadmaps/quantum/www.ibm.com/roadmaps/quantum
Acknowledgements
We express our sincere thanks to the Senior Editor of Nature Catalysis, J.-S. Völler and P. Zapletal from the University of Erlangen-Nuremberg for their constructive comments on the manuscript. We are also grateful for the computational resources provided by the e-INFRA CZ and ELIXIR-CZ projects (90254 and LM2023055), supported by the Ministry of Education, Youth and Sports of the Czech Republic, and by the National Institute for Neurology Research (LX22NPO5107), funded by the European Union—Next Generation EU. We also thank the RECETOX Research Infrastructure (No LM2023069) financed by the MEYS for a supportive background.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
J.D. is the co-founder of the biotechnology spin-off company Enantis. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Sílvia Osuna and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Damborsky, J., Kouba, P., Sivic, J. et al. Quantum computing for faster enzyme discovery and engineering. Nat Catal 8, 872–880 (2025). https://doi.org/10.1038/s41929-025-01410-w
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41929-025-01410-w