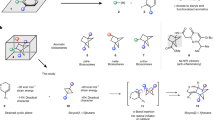
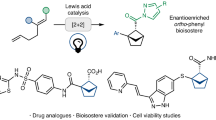
Abstract
Substituted cyclohexanes are ubiquitous motifs in bioactive molecules. Thermodynamically disfavoured substituted cyclohexane scaffolds can significantly enhance both the biological activity and pharmacokinetic properties of potential drugs. However, achieving stereoselective cross-coupling for the synthesis of these structures with precise kinetic control remains a challenge. Here we present a modular reductive cross-coupling reaction that enables the stereoselective synthesis of thermodynamically disfavoured substituted cyclohexanes, employing simple alkenes as coupling partners. Mechanistically, the exceptional stereochemistry of this reaction is governed by a Heck-type migratory insertion step. The utility of this method is also demonstrated through the concise synthesis of bioactive molecules.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All information relating to optimization studies, experimental procedures, DFT calculations, NMR spectra and high-resolution mass spectrometry are available in the Supplementary Information. All other data are available from the corresponding authors upon request. The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC) under deposition numbers CCDC 2242056 (15), 2239184 (18), 2242054 (23), 2239177 (30), 2226452 (46) and 2242066 (61). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.
References
Aldeghi, M., Malhotra, S., Selwood, D. L. & Chan, A. W. E. Two- and three-dimensional rings in drugs. Chem. Biol. Drug. Des. 83, 450–461 (2014).
Tsien, J., Hu, C., Merchant, R. R. & Qin, T. Three-dimensional saturated C(sp3)-rich bioisosteres for benzene. Nat. Rev. Chem. 8, 605–627 (2024).
Lovering, F. Escape from Flatland 2: complexity and promiscuity. MedChemComm 4, 515–519 (2013).
Cherney, E. C. et al. Conformational-analysis-guided discovery of 2,3-disubstituted pyridine IDO1 inhibitors. ACS Med. Chem. Lett. 12, 1143–1150 (2021).
Brameld, K. A., Kuhn, B., Reuter, D. C. & Stahl, M. Small molecule conformational preferences derived from crystal structure data. A medicinal chemistry focused analysis. J. Chem. Inf. Model 48, 1–24 (2008).
Subbaiah, M. A. M. & Meanwell, N. A. Bioisosteres of the phenyl ring: recent strategic applications in lead optimization and drug design. J. Med. Chem. 64, 14046–14128 (2021).
Staszewski, M. et al. Guanidine derivatives: how simple structural modification of histamine H3R antagonists has led to the discovery of potent muscarinic M2R/M4R antagonists. ACS Chem. Neurosci. 12, 2503–2519 (2021).
Hekking, K. F. W. et al. Development of potent Mcl-1 inhibitors: structural investigations on macrocycles originating from a DNA-encoded chemical library screen. J. Med. Chem. 67, 3039–3065 (2024).
Simonin, C. et al. Optimization of TRPV6 calcium channel inhibitors using a 3D ligand-based virtual screening method. Angew. Chem. Int. Ed. 54, 14748–14752 (2015).
Li, Y., Shi, H. & Yin, G. Synthetic techniques for thermodynamically disfavoured substituted six-membered rings. Nat. Rev. Chem. 8, 535–550 (2024).
Wiesenfeldt, M. P., Nairoukh, Z., Li, W. & Glorius, F. Hydrogenation of fluoroarenes: direct access to all-cis-(multi)fluorinated cycloalkanes. Science 357, 908–912 (2017).
Wiesenfeldt, M. P., Knecht, T., Schlepphorst, C. & Glorius, F. Silylarene hydrogenation: a strategic approach that enables direct access to versatile silylated saturated carbo- and heterocycles. Angew. Chem. Int. Ed. 57, 8297–8300 (2018).
Ling, L., He, Y., Zhang, X., Luo, M. & Zeng, X. Hydrogenation of (hetero)aryl boronate esters with a cyclic (alkyl)(amino)carbene–rhodium complex: direct access to cis-substituted borylated cycloalkanes and saturated heterocycles. Angew. Chem. Int. Ed. 58, 6554–6558 (2019).
Kaithal, A. et al. cis-Selective hydrogenation of aryl germanes: a direct approach to access saturated carbo- and heterocyclic germanes. J. Am. Chem. Soc. 145, 4109–4118 (2023).
Liang, Y. et al. Selective 1,4-syn-addition to cyclic 1,3-dienes via hybrid palladium catalysis. ACS Cent. Sci. 10, 1191–1200 (2024).
Miyaura, N. & Buchwald, S. L. Cross-coupling Reactions: A Practical Guide (Springer, 2002).
Jana, R., Pathak, T. P. & Sigman, M. S. Advances in transition metal (Pd,Ni,Fe)-catalyzed cross-coupling reactions using alkyl-organometallics as reaction partners. Chem. Rev. 111, 1417–1492 (2011).
Cherney, A. H., Kadunce, N. T. & Reisman, S. E. Enantioselective and enantiospecific transition-metal-catalyzed cross-coupling reactions of organometallic reagents to construct C–C bonds. Chem. Rev. 115, 9587–9652 (2015).
Choi, J. & Fu, G. C. Transition metal–catalyzed alkyl-alkyl bond formation: Another dimension in cross-coupling chemistry. Science 356, eaaf7230 (2017).
Diccianni, J. B. & Diao, T. Mechanisms of nickel-catalyzed cross-coupling reactions. Trends Chem. 1, 830–844 (2019).
Kotesova, S. & Shenvi, R. A. Inner- and outer-sphere cross-coupling of high Fsp3 fragments. Acc. Chem. Res. 56, 3089–3098 (2023).
Nakamura, M., Matsuo, K., Ito, S. & Nakamura, E. Iron-catalyzed cross-coupling of primary and secondary alkyl halides with aryl Grignard reagents. J. Am. Chem. Soc. 126, 3686–3687 (2004).
Thaler, T. et al. Highly diastereoselective Csp3–Csp2 Negishi cross-coupling with 1,2-, 1,3- and 1,4-substituted cycloalkylzinc compounds. Nat. Chem. 2, 125–130 (2010).
Mu, X., Shibata, Y., Makida, Y. & Fu, G. C. Control of vicinal stereocenters through nickel-catalyzed alkyl–alkyl cross-coupling. Angew. Chem. Int. Ed. 56, 5821–5824 (2017).
Matier, C. D., Schwaben, J., Peters, J. C. & Fu, G. C. Copper-catalyzed alkylation of aliphatic amines induced by visible light. J. Am. Chem. Soc. 139, 17707–17710 (2017).
Komeyama, K., Michiyuki, T. & Osaka, I. Nickel/cobalt-catalyzed C(sp3)–C(sp3) cross-coupling of alkyl halides with alkyl tosylates. ACS Catal. 9, 9285–9291 (2019).
Li, J., Ren, Q., Cheng, X., Karaghiosoff, K. & Knochel, P. Chromium(II)-catalyzed diastereoselective and chemoselective Csp2–Csp3 cross-couplings using organomagnesium reagents. J. Am. Chem. Soc. 141, 18127–18135 (2019).
Górski, B., Barthelemy, A.-L., Douglas, J. J., Juliá, F. & Leonori, D. Copper-catalysed amination of alkyl iodides enabled by halogen-atom transfer. Nat. Catal. 4, 623–630 (2021).
Takeuchi, D., Watanabe, K., Sogo, K. & Osakada, K. Polymerization of methylenecyclohexanes catalyzed by diimine–Pd complex. Polymers having trans- or cis-1,4- and trans-1,3-cyclohexylene groups. Organometallics 34, 3007–3011 (2015).
Zhu, D. et al. Asymmetric three-component Heck arylation/amination of nonconjugated cyclodienes. Angew. Chem. Int. Ed. 59, 5341–5345 (2020).
Li, Y. et al. Modular access to substituted cyclohexanes with kinetic stereocontrol. Science 376, 749–753 (2022).
Chen, C., Guo, W., Qiao, D. & Zhu, S. Synthesis of enantioenriched 1,2-cis disubstituted cycloalkanes by convergent NiH catalysis. Angew. Chem. Int. Ed. 62, e202308320 (2023).
Gurak, J. A.Jr, Yang, K. S., Liu, Z. & Engle, K. M.Directed, regiocontrolled hydroamination of unactivated alkenes via protodepalladation. J. Am. Chem. Soc. 138, 5805–5808 (2016).
Li, Y. & Yin, G. Nickel chain-walking catalysis: a journey to migratory carboboration of alkenes. Acc. Chem. Res. 56, 3246–3259 (2023).
Shen, Z. et al. Creating glycoside diversity through stereoselective carboboration of glycals. Nat. Commun. 15, 10167 (2024).
Yu, S., Berner, O. M. & Cook, J. M. General approach for the synthesis of indole alkaloids via the asymmetric Pictet–Spengler reaction: first enantiospecific total synthesis of (−)-corynantheidine as well as the enantiospecific total synthesis of (−)-corynantheidol, (−)-geissoschizol, and (+)-geissoschizine. J. Am. Chem. Soc. 122, 7827–7828 (2000).
Yang, F., Jin, Y. & Wang, C. Nickel-catalyzed asymmetric intramolecular reductive Heck reaction of unactivated alkenes. Org. Lett. 21, 6989–6994 (2019).
Xiao, X. & Liu, J. Progress in the synthesis of C(sp2)–C(sp3) bond by reductive Heck reactions of alkenes. Chin. J. Org. Chem. 41, 3349–3365 (2021).
Xie, J.-Q., Liang, R.-X. & Jia, Y.-X. Recent advances of catalytic enantioselective Heck reactions and reductive-Heck reactions. Chin. J. Chem. 39, 710–728 (2021).
Zhang, L. et al. Nickel-catalyzed enantioselective reductive conjugate arylation and heteroarylation via an elementary mechanism of 1,4-addition. J. Am. Chem. Soc. 144, 20249–20257 (2022).
Wang, X.-X., Lu, X., Li, Y., Wang, J.-W. & Fu, Y. Recent advances in nickel-catalyzed reductive hydroalkylation and hydroarylation of electronically unbiased alkenes. Sci. China Chem. 63, 1586–1600 (2020).
Wang, Y., He, Y. & Zhu, S. NiH-catalyzed functionalization of remote and proximal olefins: new reactions and innovative strategies. Acc. Chem. Res. 55, 3519–3536 (2022).
Zhang, Z., Bera, S., Fan, C. & Hu, X. Streamlined alkylation via nickel-hydride-catalyzed hydrocarbonation of alkenes. J. Am. Chem. Soc. 144, 7015–7029 (2022).
Perez Garcia, P. M., Di Franco, T., Orsino, A., Ren, P. & Hu, X. Nickel-catalyzed diastereoselective alkyl–alkyl Kumada coupling reactions. Org. Lett. 14, 4286–4289 (2012).
Lu, Z. & Fu, G. C. Alkyl–alkyl Suzuki cross-coupling of unactivated secondary alkyl chlorides. Angew. Chem. Int. Ed. 49, 6676–6678 (2010).
Aragón, J., Sun, S., Pascual, D., Jaworski, S. & Lloret-Fillol, J. Photoredox activation of inert alkyl chlorides for the reductive cross-coupling with aromatic alkenes. Angew. Chem. Int. Ed. 61, e202114365 (2022).
Yang, Y. et al. Practical and modular construction of C(sp3)-rich alkyl boron compounds. J. Am. Chem. Soc. 143, 471–480 (2021).
Lyon, W. L. & MacMillan, D. W. C. Expedient access to underexplored chemical space: deoxygenative C(sp3)–C(sp3) cross-coupling. J. Am. Chem. Soc. 145, 7736–7742 (2023).
Dziechciejewski, W. J., Weber, R., Sowada, O. & Boysen, M. M. K. Cycloalkene carbonitriles in rhodium-catalyzed 1,4-addition and formal synthesis of vabicaserin. Org. Lett. 17, 4132–4135 (2015).
Higuchi, R. I. et al. 4-Alkyl- and 3,4-dialkyl-1,2,3,4-tetrahydro-8-pyridono[5,6-g]quinolines: potent, nonsteroidal androgen receptor agonists. Bioorg. Med. Chem. Lett. 9, 1335–1340 (1999).
Ma, R. et al. Stereoselective synthesis of chiral hydrophenanthridines via a one-pot stepwise aza-Michael/Michael/Michael process. Org. Lett. 24, 4798–4803 (2022).
Hamilton, M. M. et al. Discovery of IACS-9779 and IACS-70465 as potent inhibitors targeting indoleamine 2,3-dioxygenase 1 (IDO1) apoenzyme. J. Med. Chem. 64, 11302–11329 (2021).
Beck, H. P. et al. Preparation of immunoregulatory agents. WO patent 2,016,073,774 (2016).
Pang, X. et al. Regiocontrolled reductive vinylation of aliphatic 1,3-dienes with vinyl triflates by nickel catalysis. J. Am. Chem. Soc. 143, 4536–4542 (2021).
Zhu, S., He, Y., Xue, Y., Chen, J. & Song, P. Nickel-catalyzed regiodivergent reductive hydroarylation of styrenes. Synlett 32, 1647–1651 (2021).
Lyu, X. et al. Intramolecular hydroamidation of alkenes enabling asymmetric synthesis of β-lactams via transposed NiH catalysis. Nat. Catal. 6, 784–795 (2023).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant no. 22122107 to G.Y. and grant no. 22401220 to Y.L.), the Fundamental Research Funds for the Central Universities (grant no. 413100070 to Y.L.), Guangdong Basic and Applied Basic Research Foundation (grant no. 2024A1515011689 to G.Y. and grant no. 2025A1515010310 to Y.L.), the National Key R&D Program of China (grant nos. 2022YFA1505100 and 2023YFA1508600 to X.Q.), the Scientific Research Innovation Capability Support Project for Young Faculty (grant no. ZYGXQNJSKYCXNLZCXM-H17 to G.Y.) and the supercomputing system in the Supercomputing Center of Wuhan University. We thank the Core Facility of Wuhan University for help with X-ray crystallographic analysis.
Author information
Authors and Affiliations
Contributions
G.Y. designed the project and directed the work. Z.S., H.S. and Y.L. performed all synthetic experiments. X.Z. and X.Q. performed all DFT calculations. G.Y., Y.L. and Z.S. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Rodrigo A Cormanich and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–9, Figs. 1–9, methods, characterization data, DFT calculations, mechanistic discussion and references.
Supplementary Data 1
CIF file of the crystal structure of compound 15.
Supplementary Data 2
CIF file of the crystal structure of compound 18.
Supplementary Data 3
CIF file of the crystal structure of compound 23.
Supplementary Data 4
CIF file of the crystal structure of compound 30.
Supplementary Data 5
CIF file of the crystal structure of compound 46.
Supplementary Data 6
CIF file of the crystal structure of compound 61.
Supplementary Data 7
Atomic coordinates of the optimized structures.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shen, Z., Shi, H., Li, Y. et al. A stereoselective reductive cross-coupling reaction with kinetic control. Nat Catal 8, 1241–1249 (2025). https://doi.org/10.1038/s41929-025-01440-4
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41929-025-01440-4