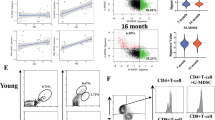
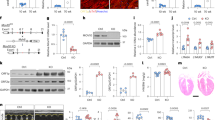
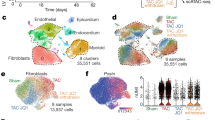
Abstract
Age-related cardiac fibrosis is a key driver of heart failure and hallmark of aging whose mechanisms remain incompletely understood. Here we show elevated succinate levels in aged mice and humans drive cardiac fibrosis by enhancing fibroblast activation and collagen production. This process is mediated through succinate-dependent succinylation of PKM2 at lysine 125, promoting its transition from tetrameric to dimeric states. Using SUCNR1–/– mice, we establish that succinate signaling through SUCNR1/GPR91 promotes PKM2 succinylation and dimerization, creating a profibrotic network associated with aging-related diastolic dysfunction. Nuclear translocation of dimeric PKM2 enables fibroblast activation through HIF-1α binding, enhancing DNA-binding affinity and upregulating fibrogenic genes. Metformin treatment suppresses fibroblast activation by reducing succinate accumulation, revealing therapeutic potential for mitigating age-related cardiac fibrosis and diastolic dysfunction. Our findings identify metabolic dysregulation as a critical target and characterize a succinate-PKM2 signaling axis whose interruption may attenuate cardiac aging.

Similar content being viewed by others
Data availability
All data supporting the findings of this study are included in the article and its Supplementary Information. The source data underlying the graphs are available in Supplementary Data files provided with this paper.
References
Amegadzie, J.E. et al. Twenty-year trends in excess costs of chronic obstructive pulmonary disease. Eur Respir J 65, 2400516 (2025).
Chen, S. et al. Current situation and progress toward the 2030 health-related sustainable development goals in China: a systematic analysis. PLoS Med. 16, e1002975 (2019).
Sau, A. et al. Artificial intelligence-enabled electrocardiogram for mortality and cardiovascular risk estimation: a model development and validation study. Lancet Digit. Health 6, e791–e802 (2024).
Fayyaz, A.U. et al. Pathophysiological insights into HFpEF from studies of human cardiac tissue. Nat. Rev. Cardiol. 22, 90–104 (2025).
Steffens, S. et al. Stimulating pro-reparative immune responses to prevent adverse cardiac remodelling: consensus document from the joint 2019 meeting of the ESC Working Groups of cellular biology of the heart and myocardial function. Cardiovasc. Res. 116, 1850–1862 (2020).
Moreira, L. M. et al. Paracrine signalling by cardiac calcitonin controls atrial fibrogenesis and arrhythmia. Nature 587, 460–465 (2020).
Maliken, B. D. et al. Gata4-dependent differentiation of c-Kit(+)-derived endothelial cells underlies artefactual cardiomyocyte regeneration in the heart. Circulation 138, 1012–1024 (2018).
Ham, P. B. III & Raju, R. Mitochondrial function in hypoxic ischemic injury and influence of aging. Prog. Neurobiol. 157, 92–116 (2017).
Li, Y. et al. SIRT3 deficiency exacerbates p53/Parkin‑mediated mitophagy inhibition and promotes mitochondrial dysfunction: Implication for aged hearts. Int. J. Mol. Med. 41, 3517–3526 (2018).
Oh, C. J. et al. Inhibition of pyruvate dehydrogenase kinase 4 ameliorates kidney ischemia-reperfusion injury by reducing succinate accumulation during ischemia and preserving mitochondrial function during reperfusion. Kidney Int. 104, 724–739 (2023).
Figueroa-Juarez, E. Uncovering the origin of oxidative damage in ischaemia-reperfusion injury in the heart. Nat. Rev. Endocrinol. 19, 560 (2023).
Littlewood-Evans, A. et al. GPR91 senses extracellular succinate released from inflammatory macrophages and exacerbates rheumatoid arthritis. J. Exp. Med. 213, 1655–1662 (2016).
Kokaia, Z. & Lindvall, O. Sensors of succinate: neural stem cell grafts fight neuroinflammation. Cell Stem Cell 22, 283–285 (2018).
Bhandari, R. & Cameron, S. J. Breaking the cycle: succinate in aortic diseases. Eur. Heart J. 42, 4386–4388 (2021).
Reddy, A. et al. pH-gated succinate secretion regulates muscle remodeling in response to exercise. Cell 183, 62–75.e17 (2020).
Tubben, A. et al. Circulating ECM proteins decorin and alpha-L-iduronidase differentiate ATTRwt-CM from ATTRwt-negative HFpEF/HFmrEF. Cardiovasc. Res. 120, 1727–1736 (2024).
Lozhkin, A. et al. Mitochondrial oxidative stress contributes to diastolic dysfunction through impaired mitochondrial dynamics. Redox Biol. 57, 102474 (2022).
Doenst, T. et al. Decreased rates of substrate oxidation ex vivo predict the onset of heart failure and contractile dysfunction in rats with pressure overload. Cardiovasc. Res. 86, 461–470 (2010).
Shook, B. A. et al. Dermal adipocyte lipolysis and myofibroblast conversion are required for efficient skin repair. Cell Stem Cell 26, 880–895.e886 (2020).
Amrute, J.M. et al. Targeting immune-fibroblast cell communication in heart failure. Nature 635, 423–433 (2024).
Kanisicak, O. et al. Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nat. Commun. 7, 12260 (2016).
Yu, Z. et al. Nutrient-sensing alteration leads to age-associated distortion of intestinal stem cell differentiating direction. Nat. Commun. 15, 9243 (2024).
Gomes, A. P. et al. Age-induced accumulation of methylmalonic acid promotes tumour progression. Nature 585, 283–287 (2020).
Wang, Z. et al. Pharmaceutical targeting of succinate dehydrogenase in fibroblasts controls bleomycin-induced lung fibrosis. Redox Biol. 46, 102082 (2021).
Frantz, S. et al. Tissue inhibitor of metalloproteinases levels in patients with chronic heart failure: an independent predictor of mortality. Eur. J. Heart Fail. 10, 388–395 (2008).
Sanada, S. et al. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J. Clin. Investig. 117, 1538–1549 (2007).
Sarhene, M. et al. Biomarkers in heart failure: the past, current and future. Heart Fail. Rev. 24, 867–903 (2019).
Januzzi, J. L., Mebazaa, A. & Di Somma, S. ST2 and prognosis in acutely decompensated heart failure: the International ST2 Consensus Panel. Am. J. Cardiol. 115, 26B–31B (2015).
Luo, M. et al. Mitigation of radiation-induced pulmonary fibrosis by small-molecule dye IR-780. Free Radic. Biol. Med. 164, 417–428 (2021).
Tennant, D. A. PK-M2 makes cells sweeter on HIF1. Cell 145, 647–649 (2011).
Traxler, L. et al. Warburg-like metabolic transformation underlies neuronal degeneration in sporadic Alzheimer’s disease. Cell Metab. 34, 1248–1263 e1246 (2022).
Luo, W. et al. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell 145, 732–744 (2011).
Chaneton, B. et al. Serine is a natural ligand and allosteric activator of pyruvate kinase M2. Nature 491, 458–462 (2012).
Anastasiou, D. et al. Pyruvate kinase M2 activators promote tetramer formation and suppress tumorigenesis. Nat. Chem. Biol. 8, 839–847 (2012).
Zhang, Z. et al. Identification of lysine succinylation as a new post-translational modification. Nat. Chem. Biol. 7, 58–63 (2011).
Gilissen, J., Jouret, F., Pirotte, B. & Hanson, J. Insight into SUCNR1 (GPR91) structure and function. Pharm. Ther. 159, 56–65 (2016).
Liu, A., Liu, Y., Zhang, W. & Ye, R. D. Structural insights into ligand recognition and activation of the succinate receptor SUCNR1. Cell Rep. 43, 114381 (2024).
Zheng, F. et al. The HIF-1alpha antisense long non-coding RNA drives a positive feedback loop of HIF-1alpha mediated transactivation and glycolysis. Nat. Commun. 12, 1341 (2021).
Loffredo, F. S. et al. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell 153, 828–839 (2013).
Chen, K. et al. Klotho Deficiency Causes Heart Aging via Impairing the Nrf2-GR Pathway. Circ. Res. 128, 492–507 (2021).
Zhou, R. P. et al. Modulators of ASIC1a and its potential as a therapeutic target for age-related diseases. Ageing Res. Rev. 83, 101785 (2023).
Chouchani, E. T. et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature 515, 431–435 (2014).
Sapieha, P. et al. The succinate receptor GPR91 in neurons has a major role in retinal angiogenesis. Nat. Med. 14, 1067–1076 (2008).
Wang, J., Yin, J., Liu, X., Liu, Y. & Jin, X. Gut commensal bacterium Bacteroides vulgatus exacerbates helminth-induced cardiac fibrosis through succinate accumulation. PLoS Pathog. 21, e1013069 (2025).
Travers, J. G., Kamal, F. A., Robbins, J., Yutzey, K. E. & Blaxall, B. C. Cardiac fibrosis: the fibroblast awakens. Circ. Res. 118, 1021–1040 (2016).
Chiao, Y.A. et al. Late-life restoration of mitochondrial function reverses cardiac dysfunction in old mice. Elife 9, e55513 (2020).
Campisi, J. & d’Adda di Fagagna, F. Cellular senescence: when bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 8, 729–740 (2007).
Demaria, M. et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev. Cell 31, 722–733 (2014).
Lopes-Paciencia, S. et al. The senescence-associated secretory phenotype and its regulation. Cytokine 117, 15–22 (2019).
Burns, P. A. & Wilson, D. J. Angiogenesis mediated by metabolites is dependent on vascular endothelial growth factor (VEGF). Angiogenesis 6, 73–77 (2003).
Noguchi, T., Inoue, H. & Tanaka, T. The M1- and M2-type isozymes of rat pyruvate kinase are produced from the same gene by alternative RNA splicing. J. Biol. Chem. 261, 13807–13812 (1986).
Regitz-Zagrosek, V. & Kararigas, G. Mechanistic pathways of sex differences in cardiovascular disease. Physiol. Rev. 97, 1–37 (2017).
Knowlton, A. A. & Lee, A. R. Estrogen and the cardiovascular system. Pharm. Ther. 135, 54–70 (2012).
Ventura-Clapier, R. et al. Sex in basic research: concepts in the cardiovascular field. Cardiovasc. Res. 113, 711–724 (2017).
Gupta, R. et al. Post-translational modifications: regulators of neurodegenerative proteinopathies. Ageing Res Rev. 68, 101336 (2021).
Black, J. C., Van Rechem, C. & Whetstine, J. R. Histone lysine methylation dynamics: establishment, regulation, and biological impact. Mol. Cell 48, 491–507 (2012).
Wang, C. et al. Role of succinylation modification in central nervous system diseases. Ageing Res Rev. 95, 102242 (2024).
Yang, Y. et al. Altered succinylation of mitochondrial proteins, APP and tau in Alzheimer’s disease. Nat. Commun. 13, 159 (2022).
Qi, H. et al. Succinylation-dependent mitochondrial translocation of PKM2 promotes cell survival in response to nutritional stress. Cell Death Dis. 10, 170 (2019).
Yang, Y. & Gibson, G. E. Succinylation links metabolism to protein functions. Neurochem. Res. 44, 2346–2359 (2019).
Magadum, A. et al. Pkm2 regulates cardiomyocyte cell cycle and promotes cardiac regeneration. Circulation 141, 1249–1265 (2020).
Tannahill, G. M. et al. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature 496, 238–242 (2013).
>Yeh, Y. H., Hsiao, H. F., Yeh, Y. C., Chen, T. W. & Li, T. K. Inflammatory interferon activates HIF-1alpha-mediated epithelial-to-mesenchymal transition via PI3K/AKT/mTOR pathway. J. Exp. Clin. Cancer Res. 37, 70 (2018).
Tong, Y. et al. SUCLA2-coupled regulation of GLS succinylation and activity counteracts oxidative stress in tumor cells. Mol. Cell 81, 2303–2316.e2308 (2021).
Cheng, X., Wang, K., Zhao, Y. & Wang, K. Research progress on post-translational modification of proteins and cardiovascular diseases. Cell Death Discov. 9, 275 (2023).
Wang, Z. et al. Cordycepin prevents radiation ulcer by inhibiting cell senescence via NRF2 and AMPK in rodents. Nat. Commun. 10, 2538 (2019).
Acknowledgements
This work was supported by the China National Postdoctoral Program for Innovative Talents (BX2021198 to Z.W.), China Postdoctoral Science Foundation (2022M712215 to Z.W.), Beijing Postdoctoral Research Foundation (2022-ZZ-009 to Z.W.), National Natural Science Foundation of China (32100939 to Z.W.), Hebei Natural Science Foundation (C2022104004 to Z.W.), Hebei Medical Science Research Project (20221909 to Z.W., 20221907 to X. Zhang and 20232039 to Z.P.), Military Traditional Chinese Medicine Service Capacity Cultivation and Enhancement Project(2023ZY013 X.C.), Military Logistics Research Project (ZLJ22J027 to X.C.), Army Medical Research Project (2023JS04 to Z.L.), Chengdu Medical College Clinical Research Fund Project (2022LHJYZD-01 to Z.J.), Open Research Topic of Sichuan Provincial Clinical Research Center for Geriatric Medicine (2022LHTD-01 to Z.J.), Chengdu Medical College Clinical Research Fund Project (2021LHJYZD-03 to Z.J.). We gratefully acknowledge Professor Xuebin Cao, our co-corresponding author, for covering the Article Processing Charges (APC).
Author information
Authors and Affiliations
Contributions
Z. Wang, D. Zhou, and X. Cao led the study conception and design; H. Shi and T. Sun participated in the design process; Z. Wang performed most of the experiments and performed the data analysis; Z. Zhang, Z. Ping, S. Yang, Y. Li, T. Jiang, and X. Zheng performed some experiments; Q. Zhang, Z. Liu, X. Zhang, Z. Jiang, L. Deng, H. Sun, and B. Wu analyzed and interpreted data from experiments; Z. Wang wrote the paper; all authors discussed the results and commented on the manuscript. Z. Wang ordered SUCNR1–/– mice from Cyagen Biosciences Inc. and expanded the colony to a scale sufficient for the present study as well as other fibrosis-related experiments requiring this mouse model within the laboratory.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Andreas Romaine and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editors: Joao Valente. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, Z., Zhang, Z., Ping, Z. et al. Succinate-driven PKM2 succinylation and dimerization accelerates age-associated cardiac fibrosis. Commun Biol (2025). https://doi.org/10.1038/s42003-025-09337-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-025-09337-5