Abstract
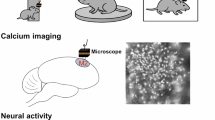
The ability to move within a given environment necessitates constant regulation of sensory and motor functions. However, intricacies of sensory-motor integration via intercortical signal correlation remain to be fully elucidated. In this study, we dissociated internally driven cortical dominance from original signals by removing the influence of behavior variables during locomotion on motorized treadmill, wheel, and disk. There were no significant differences in either original or internally driven activity across the cortex of mice during walking based on the type of track. However, the spatial pattern of internally driven cortical connectivity depended on the track type. Especially, internally driven functional connectivity during sustained locomotion on the treadmill significantly decreased only in the medial M2 regions. Thus, the maintenance of stable locomotion on a linear runway is indicative of successful internal sensory-motor integration, which is achieved through inhibitory control of M2. Our findings demonstrate that the spatial patterns of cortical functional connectivity during locomotion are altered by the gait kinematics following physical rotation of the track. Furthermore, we suggest that understanding of health and disorder related to locomotion in environmental contexts requires the consideration of internally driven activity and functional connectivity across the widefield cortex.
Similar content being viewed by others
Data availability
Quantitative data and source data supporting this study can be obtained at https://doi.org/10.5281/zenodo.18162257. Raw data files are available from K. Lee on reasonable request.
Code availability
The sICA can be downloaded from https://sccn.ucsd.edu/~arno/eeglab/auto/jader.html. The PLSR toolbox can be downloaded from https://kr.mathworks.com/products/statistics.html. The custom code for analysis supporting this study can be obtained at https://github.com/lch199912/Widefield_Code/tree/main/imaging_analysis.
References
González-Rueda, A. et al. Kinetic features dictate sensorimotor alignment in the superior colliculus. Nature 631, 378–385 (2024).
Parker, P. R. L., Brown, M. A., Smear, M. C. & Niell, C. M. Movement-related signals in sensory areas: roles in natural behavior. Trends Neurosci. 43, 581–595 (2020).
Ayaz, A. et al. Layer-specific integration of locomotion and sensory information in mouse barrel cortex. Nat. Commun. 10, 2585 (2019).
Heindorf, M., Arber, S. & Keller, G. B. Mouse motor cortex coordinates the behavioral response to unpredicted sensory feedback. Neuron 99, 1040–1054.e1045 (2018).
Rao, R. P. N. A sensory–motor theory of the neocortex. Nat. Neurosci. 27, 1221–1235 (2024).
Clancy, K. B., Orsolic, I. & Mrsic-Flogel, T. D. Locomotion-dependent remapping of distributed cortical networks. Nat. Neurosci. 22, 778–786 (2019).
Arber, S. Motor circuits in action: specification, connectivity, and function. Neuron 74, 975–989 (2012).
Omlor, W. et al. Context-dependent limb movement encoding in neuronal populations of motor cortex. Nat. Commun. 10, 4812 (2019).
Cruz, K. G. et al. Cortical-subcortical interactions in goal-directed behavior. Physiol. Rev. 103, 347–389 (2022).
Lu, L. et al. Control of locomotor speed, arousal, and hippocampal theta rhythms by the nucleus incertus. Nat. Commun. 11, 262 (2020).
Melzer, S. et al. Distinct corticostriatal GABAergic neurons modulate striatal output neurons and motor activity. Cell Rep. 19, 1045–1055 (2017).
Barthas, F. & Kwan, A. C. Secondary motor cortex: where ‘sensory’ meets ‘motor’ in the rodent frontal cortex. Trends Neurosci. 40, 181–193 (2017).
Leinweber, M., Ward, D. R., Sobczak, J. M., Attinger, A. & Keller, G. B. A sensorimotor circuit in mouse cortex for visual flow predictions. Neuron 95, 1420–1432.e1425 (2017).
West, S. L., Gerhart, M. L. & Ebner, T. J. Wide-field calcium imaging of cortical activation and functional connectivity in externally- and internally-driven locomotion. Nat. Commun. 15, 7792 (2024).
Sun, G. et al. Neural representation of self-initiated locomotion in the secondary motor cortex of mice across different environmental contexts. Commun. Biol. 8, 725 (2025).
Dana, H. et al. Thy1-GCaMP6 transgenic mice for neuronal population imaging in vivo. PLoS ONE 9, e108697 (2014).
Kim, T. H. et al. Long-term optical access to an estimated one million neurons in the live mouse cortex. Cell Rep. 17, 3385–3394 (2016).
Cardoso, J.-F. High-order contrasts for independent component analysis. Neural Comput. 11, 157–192 (1999).
Wang, Q. et al. The Allen Mouse Brain Common Coordinate Framework: a 3D Reference Atlas. Cell 181, 936–953.e920 (2020).
Vélez-Fort, M., Cossell, L., Porta, L., Clopath, C. & Margrie, T. W. Motor and vestibular signals in the visual cortex permit the separation of self versus externally generated visual motion. Cell 188, 2175–2189.e2115 (2025).
Mao, D., Molina, L. A., Bonin, V. & McNaughton, B. L. Vision and locomotion combine to drive path integration sequences in mouse retrosplenial cortex. Curr. Biol. 30, 1680–1688.e1684 (2020).
Dadarlat, M. C. & Stryker, M. P. Locomotion enhances neural encoding of visual stimuli in mouse V1. J. Neurosci. 37, 3764 (2017).
West, S. L. et al. Wide-field calcium imaging of dynamic cortical networks during locomotion. Cereb. Cortex 32, 2668–2687 (2021).
Aydın, Ç, Couto, J., Giugliano, M., Farrow, K. & Bonin, V. Locomotion modulates specific functional cell types in the mouse visual thalamus. Nat. Commun. 9, 4882 (2018).
Vinck, M., Batista-Brito, R., Knoblich, U. & Cardin, J. A. Arousal and locomotion make distinct contributions to cortical activity patterns and visual encoding. Neuron 86, 740–754 (2015).
McGinley, M. atthewJ., David, S. tephenV., McCormick & David, A. Cortical membrane potential signature of optimal states for sensory signal detection. Neuron 87, 179–192 (2015).
Olson, J. M., Li, J. K., Montgomery, S. E. & Nitz, D. A. Secondary motor cortex transforms spatial information into planned action during navigation. Curr. Biol. 30, 1845–1854.e1844 (2020).
Keshavarzi, S. et al. Multisensory coding of angular head velocity in the retrosplenial cortex. Neuron 110, 532–543.e539 (2022).
Fischer, L. F., Mojica Soto-Albors, R., Buck, F. & Harnett, M. T. Representation of visual landmarks in retrosplenial cortex. eLife 9, e51458 (2020).
Sun, H. et al. Conjunctive processing of spatial border and locomotion in retrosplenial cortex during spatial navigation. J. Physiol. 602, 5017–5038 (2024).
Qadir, H. et al. The mouse claustrum synaptically connects cortical network motifs. Cell Rep. 41, 111860 (2022).
Yamawaki, N., Radulovic, J. & Shepherd, G. M. G. A corticocortical circuit directly links retrosplenial cortex to M2 in the mouse. J. Neurosci. 36, 9365 (2016).
Lazari, A. et al. The mouse motor system contains multiple premotor areas and partially follows human organizational principles. Cell Rep. 43, 114191 (2024).
Reep, R. L. & Corwin, J. V. Topographic organization of the striatal and thalamic connections of rat medial agranular cortex. Brain Res. 841, 43–52 (1999).
Allen, W. E. et al. Global representations of goal-directed behavior in distinct cell types of mouse neocortex. Neuron 94, 891–907.e896 (2017).
Makino, H. et al. Transformation of cortex-wide emergent properties during motor learning. Neuron 94, 880–890.e888 (2017).
Yoshida, E. et al. Whether or not to act is determined by distinct signals from motor thalamus and orbitofrontal cortex to secondary motor cortex. Nat. Commun. 16, 3106 (2025).
Augusto, E. et al. Secondary motor cortex tracks decision value during the learning of a non-instructed task. Cell Rep. 44, 115152 (2025).
Zamani Esfahlani, F. et al. High-amplitude cofluctuations in cortical activity drive functional connectivity. Proc. Natl. Acad. Sci. USA 117, 28393–28401 (2020).
Sun, Q. et al. A whole-brain map of long-range inputs to GABAergic interneurons in the mouse medial prefrontal cortex. Nat. Neurosci. 22, 1357–1370 (2019).
Holey, B. E. & Schneider, D. M. Sensation and expectation are embedded in mouse motor cortical activity. Cell Rep. 43, 114396 (2024).
Schneider, D. M., Nelson, A. & Mooney, R. A synaptic and circuit basis for corollary discharge in the auditory cortex. Nature 513, 189–194 (2014).
Gallero-Salas, Y. et al. F. Sensory and behavioral components of neocortical signal flow in discrimination tasks with short-term memory. Neuron 109, 135–148 (2021).
Mohr, H. et al. Integration and segregation of large-scale brain networks during short-term task automatization. Nat. Commun. 7, 13217 (2016).
Ghanbari, L. et al. Craniobot: A computer numerical controlled robot for cranial microsurgeries. Sci. Rep. 9, 1023 (2019).
Warren, R. A. et al. A rapid whisker-based decision underlying skilled locomotion in mice. eLife 10, e63596 (2021).
Aljovic, A. et al. A deep learning-based toolbox for Automated Limb Motion Analysis (ALMA) in murine models of neurological disorders. Commun. Biol. 5, 131 (2022).
Mathis, A. et al. DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nat. Neurosci. 21, 1281–1289 (2018).
Syeda, A. et al. Facemap: a framework for modeling neural activity based on orofacial tracking. Nat. Neurosci. 27, 187–195 (2024).
de Jong, S. SIMPLS: an alternative approach to partial least squares regression. Chemom. Intell. Lab. Syst. 18, 251–263 (1993).
Genovese, C. R., Lazar, N. A. & Nichols, T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage 15, 870–878 (2002).
Acknowledgements
We thank S.L. West and T.J. Ebner for analytical assistance with widefield imaging. This work was supported in part by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (RS-2021-NR065783, RS-2025-16903034), in part by the “DGIST intramural grant” (25-IRJoint-03, 25-HRHR-02).
Author information
Authors and Affiliations
Contributions
Study conception and design: K.L., G.L., H.S., C.H.L.; Experiments performing and data collection: C.H.L.; Visualization and data analysis: K.L., C.H.L., G.L., H.S.; Funding acquisition: K.L.; Project administration: K.L.; Supervision: K.L.; Results were discussed and interpreted by: K.L., G.L., H.S., C.H.L.; Writing—original draft: K.L., C.H.L., G.L., H.S.; Writing—review and editing: K.L., G.L., H.S.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary handling editor: Jasmine Pan.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lee, C.H., Lee, G., Song, H. et al. Widefield cortical activity and functional connectivity during motorized locomotion. Commun Biol (2026). https://doi.org/10.1038/s42003-026-09541-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-026-09541-x