Abstract
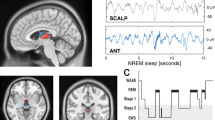
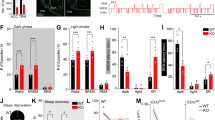
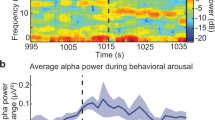
Thalamic neurons discharge tonically during wakefulness and rapid-eye-movement (REM) sleep, but switch to burst firing during non-REM (NREM) sleep. It has been hypothesized that NREM thalamic bursts do not serve as a cortical “wake-up” signal due to their periodic and synchronized nature. Here, we analyze the simultaneously recorded polysomnographic signals, field potentials, and spiking activity of neurons in the ventral anterior and centromedian thalamic nuclei of two female non-human primates during naturally occurring vigilance states. These nuclei receive GABAergic output from the basal ganglia, with discharge rate decreasing during NREM sleep. Despite this reduction in inhibitory input, NREM bursting increases significantly as reported for glutamate-driven thalamic nuclei. The NREM bursts are neither periodic nor tightly synchronized. However, delta and sleep-spindle EEG activity and thalamic field potentials time-locked to burst onset during NREM sleep markedly differ from those observed during wakefulness and REM sleep. These results suggest that the basal ganglia modulate, rather than drive, their thalamic targets. Additionally, unique state-dependent thalamocortical dynamics, rather than the periodicity or tight synchrony of the thalamic bursts, are sufficient to account for why NREM thalamic bursts do not awaken the cortex.
Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the paper as a Supplementary Data. Other data will be available from the corresponding authors upon reasonable request.
Code availability
We provided the code related to the figures of this paper as a Supplementary File. MATLAB code will also be available from the corresponding authors upon reasonable request. Please note that the code used in this study was developed by the researchers for data analysis and visualization. It is intended for research purposes and may not meet professional coding standards.
References
Crunelli, V., Errington, A. C., Hughes, S. W. & Tóth, T. I. The thalamic low-threshold Ca2+ potential: a key determinant of the local and global dynamics of the slow (< 1 Hz) sleep oscillation in thalamocortical networks. Philos. Trans. R. Soc. A Math., Phys. Eng. Sci. 369, 3820–3839 (2011).
Herrera, C. G. & Tarokh, L. A thalamocortical perspective on sleep spindle alterations in neurodevelopmental disorders. Curr. Sleep. Med. Rep. 10, 103–118 (2024).
Schreiner, T., Kaufmann, E., Noachtar, S., Mehrkens, J.-H. & Staudigl, T. The human thalamus orchestrates neocortical oscillations during NREM sleep. Nat. Commun. 13, 5231 (2022).
Gent, T. C., Bandarabadi, M., Herrera, C. G. & Adamantidis, A. R. Thalamic dual control of sleep and wakefulness. Nat. Neurosci. 21, 974–984 (2018).
Jones, E. The core and matrix of thalamic organization. Neuroscience 85, 331–345 (1998).
Usrey, W. M. & Sherman, S. M. The Cerebral Cortex and Thalamus (Oxford Univ. Press, 2024).
Sherman, S. M. & Usrey, W. M. A reconsideration of the core and matrix classification of thalamocortical projections. J. Neurosci. 44,1–4 (2024).
Sherman, S. M. & Guillery, R. W. Exploring the Thalamus and Its Role in Cortical Function (MIT Press, 2006).
Halassa, M. M. The Thalamus (Cambridge Univ. Press, 2022).
Jones, E. G. The Thalamus (Cambridge Univ. Press, 2007).
Shine, J. M. The thalamus integrates the macrosystems of the brain to facilitate complex, adaptive brain network dynamics. Prog. Neurobiol. 199, 101951 (2021).
Weyand, T. G., Boudreaux, M. & Guido, W. Burst and tonic response modes in thalamic neurons during sleep and wakefulness. J. Neurophysiol. 85, 1107–1118 (2001).
McCarley, R. W., Benoit, O. & Barrionuevo, G. Lateral geniculate nucleus unitary discharge in sleep and waking: state- and rate-specific aspects. J. Neurophysiol. 50, 798–818 (1983).
Glenn, L. & Steriade, M. Discharge rate and excitability of cortically projecting intralaminar thalamic neurons during waking and sleep states. J. Neurosci. 2, 1387–1404 (1982).
Sherman, S. M. A wake-up call from the thalamus. Nat. Neurosci. 4, 344–346 (2001).
Ramcharan, E. J., Gnadt, J. W. & Sherman, S. M. Burst and tonic firing in thalamic cells of unanesthetized, behaving monkeys. Vis. Neurosci. 17, 55–62 (2000).
Lisman, J. E. Bursts as a unit of neural information: making unreliable synapses reliable. Trends Neurosci. 20, 38–43 (1997).
Guido, W., Lu, S.-M., Vaughan, J., Godwin, D. W. & Sherman, S. M. Receiver operating characteristic (ROC) analysis of neurons in the cat’s lateral geniculate nucleus during tonic and burst response mode. Vis. Neurosci. 12, 723–741 (1995).
Lüthi, A. & McCormick, D. A. Periodicity of thalamic synchronized oscillations: the role of Ca2+-mediated upregulation of Ih. Neuron 20, 553–563 (1998).
Jahnsen, H. & Llinás, R. Ionic basis for the electro-responsiveness and oscillatory properties of guinea-pig thalamic neurones in vitro. J. Physiol. 349, 227–247 (1984).
Albin, R. L., Young, A. B. & Penney, J. B. The functional anatomy of basal ganglia disorders. Trends Neurosci. 12, 366–375 (1989).
Bergman, H., Wichmann, T. & DeLong, M. R. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science 249, 1436–1438 (1990).
Bosch-Bouju, C., Hyland, B. I. & Parr-Brownlie, L. C. Motor thalamus integration of cortical, cerebellar and basal ganglia information: implications for normal and parkinsonian conditions. Front. Comput. Neurosci. 7, 163 (2013).
Mizrahi-Kliger, A. D., Kaplan, A., Israel, Z. & Bergman, H. Desynchronization of slow oscillations in the basal ganglia during natural sleep. Proc. Natl. Acad. Sci. USA 115, E4274–E4283 (2018).
Redinbaugh, M. J. et al. Thalamus modulates consciousness via layer-specific control of cortex. Neuron 106, 66–75. e12 (2020).
Llinás, R. R. & Steriade, M. Bursting of thalamic neurons and states of vigilance. J. Neurophysiol. 95, 3297–3308 (2006).
Bar-Gad, I., Ritov, Y., Vaadia, E. & Bergman, H. Failure in identification of overlapping spikes from multiple neuron activity causes artificial correlations. J. Neurosci. Methods 107, 1–13 (2001).
Perkel, D. H., Gerstein, G. L. & Moore, G. P. Neuronal spike trains and stochastic point processes: II. Simultaneous spike trains. Biophys. J. 7, 419–440 (1967).
Zirh, T., Lenz, F., Reich, S. & Dougherty, P. Patterns of bursting occurring in thalamic cells during Parkinsonian tremor. Neuroscience 83, 107–121 (1998).
Rivlin-Etzion, M., Ritov, Y. A., Heimer, G., Bergman, H. & Bar-Gad, I. Local shuffling of spike trains boosts the accuracy of spike train spectral analysis. J. Neurophysiol. 95, 3245–3256 (2006).
Cox, K. M., Kase, D., Znati, T. & Turner, R. S. Detecting rhythmic spiking through the power spectra of point process model residuals. J. Neural Eng. 21, 046041 (2024).
Heimer, G., Bar-Gad, I., Goldberg, J. A. & Bergman, H. Synchronization of pallidal activity in the MPTP primate model of Parkinsonism is not limited to oscillatory activity. Basal Ganglia VII, 29–34 (2002).
Wong, R. O., Meister, M. & Shatz, C. J. Transient period of correlated bursting activity during development of the mammalian retina. Neuron 11, 923–938 (1993).
Nir, Y. & de Lecea, L. Sleep and vigilance states: embracing spatiotemporal dynamics. Neuron 111, 1998–2011 (2023).
dos Santos Lima, G. Z. et al. Hippocampal and cortical communication around micro-arousals in slow-wave sleep. Sci. Rep. 9, 5876 (2019).
Logothetis, N. K., Kayser, C. & Oeltermann, A. In vivo measurement of cortical impedance spectrum in monkeys: implications for signal propagation. Neuron 55, 809–823 (2007).
Marmor, O. et al. Local vs. volume conductance activity of field potentials in the human subthalamic nucleus. J. Neurophysiol. 117, 2140–2151 (2017).
Churchland, M. M. & Shenoy, K. V. Preparatory activity and the expansive null-space. Nat. Rev. Neurosci. 25, 213–236 (2024).
Vishwanath, A., Bartlett, M. J., Falk, T. & Cowen, S. L. Decoupling of motor cortex to movement in Parkinson’s dyskinesia rescued by sub-anaesthetic ketamine. Brain 148, 2135–2150 (2025).
Ben-Ari, Y. The GABA excitatory/inhibitory developmental sequence: a personal journey. Neuroscience 279, 187–219 (2014).
Mink, J. W. The basal ganglia: focused selection and inhibition of competing motor programs. Prog. Neurobiol. 50, 381–425 (1996).
Schwab, B. C. et al. Neural activity during a simple reaching task in macaques is counter to gating and rebound in basal ganglia–thalamic communication. PLoS Biol. 18, e3000829 (2020).
Goldberg, J. H., Farries, M. A. & Fee, M. S. Basal ganglia output to the thalamus: still a paradox. Trends Neurosci. 36, 695–705 (2013).
Adler, A. et al. Neurons in both pallidal segments change their firing properties similarly prior to closure of the eyes. J. Neurophysiol. 103, 346–359 (2010).
Timofeev, I., Grenier, F. & Steriade, M. Impact of intrinsic properties and synaptic factors on the activity of neocortical networks in vivo. J. Physiol. Paris 94, 343–355 (2000).
Crunelli, V. et al. Dual function of thalamic low-vigilance state oscillations: rhythm-regulation and plasticity. Nat. Rev. Neurosci. 19, 107–118 (2018).
Steriade, M., Contreras, D. & Amzica, F. Synchronized sleep oscillations and their paroxysmal developments. Trends Neurosci. 17, 201–207 (1994).
Contreras, D., Destexhe, A. & Steriade, M. Intracellular and computational characterization of the intracortical inhibitory control of synchronized thalamic inputs in vivo. J. Neurophysiol. 78, 335–350 (1997).
Steriade, M., McCormick, D. A. & Sejnowski, T. J. Thalamocortical oscillations in the sleeping and aroused brain. Science 262, 679–685 (1993).
Nir, Y. & Tononi, G. Dreaming and the brain: from phenomenology to neurophysiology. Trends Cogn. Sci. 14, 88–100 (2010).
Steriade, M. & McCarley, R. W. Neuronal activities in brainstem and basal forebrain structures controlling waking and sleep states. In Brain Control of Wakefulness Sleep 381–416 (Springer, Boston, MA, 2005).
Mizrahi-Kliger, A. D., Kaplan, A., Israel, Z., Deffains, M. & Bergman, H. Basal ganglia beta oscillations during sleep underlie Parkinsonian insomnia. Proc. Natl. Acad. Sci. USA 117, 17359–17368 (2020).
Hsieh, K.-C., Robinson, E. L. & Fuller, C. A. Sleep architecture in unrestrained rhesus monkeys (Macaca mulatta) synchronized to 24-hour light-dark cycles. Sleep 31, 1239–1250 (2008).
Reite, M., Rhodes, J., Kavan, E. & Adey, W. Normal sleep patterns in macaque monkey. Arch. Neurol. 12, 133–144 (1965).
Hagenauer, M. H. & Lee, T. M. Adolescent sleep patterns in humans and laboratory animals. Hormones Behav. 64, 270–279 (2013).
Hirshkowitz, M. Normal human sleep: an overview. Med. Clin. 88, 551–565 (2004).
Scala, F. et al. Phenotypic variation of transcriptomic cell types in mouse motor cortex. Nature 598, 144–150 (2021).
Council, N. R. et al. Guide for the Care and Use of Laboratory Animals 8th edn (National Academies Press, 2011).
Robinson, D. A. A method of measuring eye movemnent using a scieral search coil in a magnetic field. IEEE Trans. Biomed. Electron. 10, 137–145 (1963).
Glowinsky, S., Israel, Z., Heymann, S. & Bergman, H. Divide and conquer: automatic detection of the thalamus to empower DBS physiological navigation to the subthalamic region. IEEE Trans. Neural Syst. Rehabil. Eng. 33, 2672–2683 (2025).
Guang, J. et al. Toward asleep DBS: cortico-basal ganglia spectral and coherence activity during interleaved propofol/ketamine sedation mimics NREM/REM sleep activity. npj Parkinsons. Dis. 7, 67 (2021).
Goshtasby, A. Image registration by local approximation methods. Image Vis. Comput. 6, 255–261 (1988).
Joshua, M., Elias, S., Levine, O. & Bergman, H. Quantifying the isolation quality of extracellularly recorded action potentials. J. Neurosci. Methods 163, 267–282 (2007).
Cotterill, E. & Eglen, S. Burst detection methods. In In Vitro Neuronal Networks. Advances in Neurobiology (eds Chiappalone, M. et al.) Vol 22, 185–206 (Springer, Cham, 2019).
Legendy, C. & Salcman, M. Bursts and recurrences of bursts in the spike trains of spontaneously active striate cortex neurons. J. Neurophysiol. 53, 926–939 (1985).
Acknowledgements
The authors would like to thank Uri Werner-Reiss, PhD, for his valuable support with the surgical procedures and all aspects of monkey care, Tamar Ravins Yaish, DMD, and the HUJI-ELSC animal facility team for their assistance. We thank Ad Aertsen for the fruitful discussion of correlation analysis and the association index, and Andy Horn, Jackie Schiller, Pnina Rapel, Aric Agmon, and Yuval Nir for their discussions and comments on early versions of the manuscript. We acknowledge the use of large language model (LLM) tools for linguistic editing to improve the clarity and grammar of this manuscript; no scientific content was generated by these tools. This study is supported by grants from the ISF Breakthrough Research program (Grant No.: 1738/22) and the Collaborative Research Center TRR295, Germany (Project number 424778381) to HB.
Author information
Authors and Affiliations
Contributions
J.G. and H.B. conceived the research and designed the experiments. Z.I., D.W., and A.R. performed the surgical procedure. X.L. and J.G. supported the surgical procedure. They also performed the experiments, including electrophysiological and behavioral recordings, analyzed the data, and conducted the statistical analysis. X.L., J.G., and H.B. prepared the figures and wrote the manuscript. H.B. supervised the work. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Biology thanks Takeshi Kanda, Joaquín González, and the other anonymous reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Benjamin Bessieres.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, X., Guang, J., Israel, Z. et al. Entrained cortical delta–spindle activity, not periodic synchrony, prevents arousal by NREM thalamic bursts. Commun Biol (2026). https://doi.org/10.1038/s42003-026-09565-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-026-09565-3