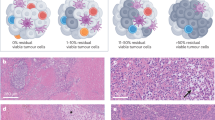
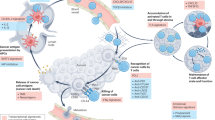
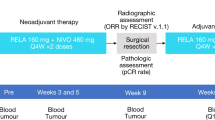
Abstract
Neoadjuvant immunotherapy is rapidly changing the treatment landscape for many tumor types. The superiority of neoadjuvant compared to adjuvant immunotherapy has now been established in both preclinical studies and clinical trials. Neoadjuvant immunotherapy, either as monotherapy or in combination with other immune checkpoint inhibitors or other agents, has become a standard of care for several cancer types, while many new indications are expected. Future research should focus on determining the benefit of treatment combinations versus monotherapy and the contribution of adjuvant after neoadjuvant (or perioperative) treatment versus neoadjuvant treatment alone as well as on identifying predictive biomarkers of response.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Boutros, A. et al. The treatment of advanced melanoma: current approaches and new challenges. Crit. Rev. Oncol. Hematol. 196, 104276 (2024).
Powles, T. et al. ESMO Clinical Practice Guideline interim update on first-line therapy in advanced urothelial carcinoma. Ann. Oncol. 35, 485–490 (2024).
Hendriks, L. E. et al. Non-oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 34, 358–376 (2023).
Patruni, S., Fayyaz, F., Bien, J., Phillip, T. & King, D. A. Immunotherapy in the management of esophagogastric cancer: a practical review. JCO Oncol. Pract. 19, 107–115 (2023).
Andre, T. et al. Nivolumab plus ipilimumab in microsatellite-instability-high metastatic colorectal cancer. N. Engl. J. Med. 391, 2014–2026 (2024).
Tran, J. & Ornstein, M. C. Clinical review on the management of metastatic renal cell carcinoma. JCO Oncol. Pract. 18, 187–196 (2022).
Cortes, J. et al. Pembrolizumab plus chemotherapy in advanced triple-negative breast cancer. N. Engl. J. Med. 387, 217–226 (2022).
Orland, M. D., Ullah, F., Yilmaz, E. & Geiger, J. L. Immunotherapy for head and neck squamous cell carcinoma: present and future approaches and challenges. JCO Oncol. Pract. 20, 1588–1595 (2024).
D’Angelo, S. P. et al. First-line avelumab treatment in patients with metastatic Merkel cell carcinoma: 4-year follow-up from part B of the JAVELIN Merkel 200 study. ESMO Open 9, 103461 (2024).
Dall’Olio, F. G. et al. Tumour burden and efficacy of immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol. 19, 75–90 (2022).
Haynes, N. M., Chadwick, T. B. & Parker, B. S. The complexity of immune evasion mechanisms throughout the metastatic cascade. Nat. Immunol. 25, 1793–1808 (2024).
Shrotriya, S., Walsh, D., Bennani-Baiti, N., Thomas, S. & Lorton, C. C-reactive protein is an important biomarker for prognosis tumor recurrence and treatment response in adult solid tumors: a systematic review. PLoS ONE 10, e0143080 (2015).
Le Bourgeois, T. et al. Targeting T cell metabolism for improvement of cancer immunotherapy. Front. Oncol. 8, 237 (2018).
Eggermont, A. M. M. et al. Five-year analysis of adjuvant pembrolizumab or placebo in stage III melanoma. NEJM Evid. 1, EVIDoa2200214 (2022).
Larkin, J. et al. Adjuvant nivolumab versus ipilimumab in resected stage III/IV melanoma: 5-year efficacy and biomarker results from CheckMate 238. Clin. Cancer Res. 29, 3352–3361 (2023).
Luke, J. J. et al. Pembrolizumab versus placebo as adjuvant therapy in resected stage IIB or IIC melanoma: final analysis of distant metastasis-free survival in the phase III KEYNOTE-716 study. J. Clin. Oncol. 42, 1619–1624 (2024).
Tang, W. F. et al. Adjuvant immunotherapy in early-stage resectable non-small cell lung cancer: a new milestone. Front. Oncol. 13, 1063183 (2023).
Kelly, R. J. et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. N. Engl. J. Med. 384, 1191–1203 (2021).
Choueiri, T. K. et al. Overall survival with adjuvant pembrolizumab in renal-cell carcinoma. N. Engl. J. Med. 390, 1359–1371 (2024).
Bajorin, D. F. et al. Adjuvant nivolumab versus placebo in muscle-invasive urothelial carcinoma. N. Engl. J. Med. 384, 2102–2114 (2021).
Dubsky, P. et al. Breast conservation and axillary management after primary systemic therapy in patients with early-stage breast cancer: the Lucerne toolbox. Lancet Oncol. 22, e18–e28 (2021).
Rödel, C. et al. Combined-modality treatment and selective organ preservation in invasive bladder cancer: long-term results. J. Clin. Oncol. 20, 3061–3071 (2002).
Garcia-Aguilar, J. et al. Organ preservation in patients with rectal adenocarcinoma treated with total neoadjuvant therapy. J. Clin. Oncol. 40, 2546–2556 (2022).
von Minckwitz, G. et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J. Clin. Oncol. 30, 1796–1804 (2012).
Rosenblatt, R. et al. Pathologic downstaging is a surrogate marker for efficacy and increased survival following neoadjuvant chemotherapy and radical cystectomy for muscle-invasive urothelial bladder cancer. Eur. Urol. 61, 1229–1238 (2012).
Petrelli, F. et al. Correlation of pathologic complete response with survival after neoadjuvant chemotherapy in bladder cancer treated with cystectomy: a meta-analysis. Eur. Urol. 65, 350–357 (2014).
Zwart, W. H. et al. Oncological outcomes after a pathological complete response following total neoadjuvant therapy or chemoradiotherapy for high-risk locally advanced rectal cancer in the RAPIDO trial. Eur. J. Cancer 204, 114044 (2024).
Masuda, N. et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N. Engl. J. Med. 376, 2147–2159 (2017).
von Minckwitz, G. et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N. Engl. J. Med. 380, 617–628 (2019).
Lordick, F. & Gockel, I. Chances, risks and limitations of neoadjuvant therapy in surgical oncology. Innov. Surg. Sci. 1, 3–11 (2016).
Versluis, J. M., Long, G. V. & Blank, C. U. Learning from clinical trials of neoadjuvant checkpoint blockade. Nat. Med. 26, 475–484 (2020).
Liu, J. et al. Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic disease. Cancer Discov. 6, 1382–1399 (2016).
Cascone, T. et al. Superior efficacy of neoadjuvant compared to adjuvant immune checkpoint blockade in non-small cell lung cancer. Cancer Res. 78, 1719 (2018).
Kaptein, P. et al. Addition of interleukin-2 overcomes resistance to neoadjuvant CTLA4 and PD1 blockade in ex vivo patient tumors. Sci. Transl. Med. 14, eabj9779 (2022).
Pardoll, D. M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264 (2012).
Chen, D. S. & Mellman, I. Oncology meets immunology: the cancer–immunity cycle. Immunity 39, 1–10 (2013).
Topalian, S. L., Taube, J. M. & Pardoll, D. M. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science 367, eaax0182 (2020).
Blank, C. U. et al. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat. Med. 24, 1655–1661 (2018).
Topalian, S. L. et al. Neoadjuvant immune checkpoint blockade: a window of opportunity to advance cancer immunotherapy. Cancer Cell 41, 1551–1566 (2023).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (2009).
Tetzlaff, M. T. et al. Pathological assessment of resection specimens after neoadjuvant therapy for metastatic melanoma. Ann. Oncol. 29, 1861–1868 (2018).
Hieken, T. J. et al. Neoadjuvant immunotherapy in melanoma: the paradigm shift. Am. Soc. Clin. Oncol. Educ. Book 43, e390614 (2023).
US Food and Drug Administration. Pathological Complete Response in Neoadjuvant Treatment of High-Risk Early-Stage Breast Cancer: Use as an Endpoint to Support Accelerated Approval. FDA-2012-D-0432 (FDA, 2020).
Menzies, A. M. et al. Pathological response and survival with neoadjuvant therapy in melanoma: a pooled analysis from the International Neoadjuvant Melanoma Consortium (INMC). Nat. Med. 27, 301–309 (2021).
Forde, P. M. et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N. Engl. J. Med. 378, 1976–1986 (2018).
Vos, J. L. et al. Neoadjuvant immunotherapy with nivolumab and ipilimumab induces major pathological responses in patients with head and neck squamous cell carcinoma. Nat. Commun. 12, 7348 (2021).
Blank, C. U. et al. Neoadjuvant nivolumab and ipilimumab in resectable stage III melanoma. N. Engl. J. Med. 391, 1696–1708 (2024).
Moschos, S. J. et al. Neoadjuvant treatment of regional stage IIIB melanoma with high-dose interferon alfa-2b induces objective tumor regression in association with modulation of tumor infiltrating host cellular immune responses. J. Clin. Oncol. 24, 3164–3171 (2006).
Tarhini, A. et al. Neoadjuvant ipilimumab (3 mg/kg or 10 mg/kg) and high dose IFN-α2b in locally/regionally advanced melanoma: safety, efficacy and impact on T-cell repertoire. J. Immunother. Cancer 6, 112 (2018).
Tarhini, A. A. et al. Immune monitoring of the circulation and the tumor microenvironment in patients with regionally advanced melanoma receiving neoadjuvant ipilimumab. PLoS ONE 9, e87705 (2014).
Gao, J. et al. VISTA is an inhibitory immune checkpoint that is increased after ipilimumab therapy in patients with prostate cancer. Nat. Med. 23, 551–555 (2017).
Tarhini, A. A. Neoadjuvant therapy for melanoma: a promising therapeutic approach and an ideal platform in drug development. Am. Soc. Clin. Oncol. Educ. Book 35, e535–e542 (2015).
Blank, C. et al. (Neo-)adjuvant ipilimumab + nivolumab (IPI + NIVO) in palpable stage 3 melanoma — initial data from the OpACIN trial. Ann. Oncol. 27, vi575 (2016).
Hoeijmakers, L. L., Reijers, I. L. M. & Blank, C. U. Biomarker-driven personalization of neoadjuvant immunotherapy in melanoma. Cancer Discov. 13, 2319–2338 (2023).
Amaria, R. N. et al. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat. Med. 24, 1649–1654 (2018).
Rozeman, E. A. et al. Identification of the optimal combination dosing schedule of neoadjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma (OpACIN-neo): a multicentre, phase 2, randomised, controlled trial. Lancet Oncol. 20, 948–960 (2019).
Najjar, Y. G. et al. Neoadjuvant pembrolizumab and high-dose IFNα-2b in resectable regionally advanced melanoma. Clin. Cancer Res. 27, 4195–4204 (2021).
Long, G. V. et al. Neoadjuvant pembrolizumab, dabrafenib and trametinib in BRAFV600-mutant resectable melanoma: the randomized phase 2 NeoTrio trial. Nat. Med. 30, 2540–2548 (2024).
Patel, S. P. et al. Neoadjuvant-adjuvant or adjuvant-only pembrolizumab in advanced melanoma. N. Engl. J. Med. 388, 813–823 (2023).
Lucas, M. W. et al. LBA42 Distant metastasis-free survival of neoadjuvant nivolumab plus ipilimumab versus adjuvant nivolumab in resectable, macroscopic stage III melanoma: the NADINA trial. Ann. Oncol. 35, S1233–S1234 (2024).
Amaria, R. N. et al. Neoadjuvant relatlimab and nivolumab in resectable melanoma. Nature 611, 155–160 (2022).
Long, G. V. et al. 1082O KEYMAKER-U02 substudy 02C: neoadjuvant pembrolizumab (pembro) and investigational agents followed by adjuvant pembro for stage IIIB–D melanoma. Ann. Oncol. 35, S712–S713 (2024).
Ascierto, P. et al. Efficacy and safety of triplet nivolumab, relatlimab, and ipilimumab (NIVO + RELA + IPI) in advanced melanoma: results from RELATIVITY-048. J. Clin. Oncol. 42, 9504 (2024).
Ayers, M. et al. IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade. J. Clin. Invest. 127, 2930–2940 (2017).
Reijers, I. L. M. et al. IFN-γ signature enables selection of neoadjuvant treatment in patients with stage III melanoma. J. Exp. Med. 220, e20221952 (2023).
Lucas, M. W., Versluis, J. M., Rozeman, E. A. & Blank, C. U. Personalizing neoadjuvant immune-checkpoint inhibition in patients with melanoma. Nat. Rev. Clin. Oncol. 20, 408–422 (2023).
Rosner, S. et al. Five-year clinical outcomes after neoadjuvant nivolumab in resectable non-small cell lung cancer. Clin. Cancer Res. 29, 705–710 (2023).
Gao, S. et al. Neoadjuvant PD-1 inhibitor (sintilimab) in NSCLC. J. Thorac. Oncol. 15, 816–826 (2020).
Besse, B. et al. 1215O - SC Neoadjuvant atezolizumab (A) for resectable non-small cell lung cancer (NSCLC): results from the phase II PRINCEPS trial. Ann. Oncol. 31, S794–S795 (2020).
Cascone, T. et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: the phase 2 randomized NEOSTAR trial. Nat. Med. 27, 504–514 (2021).
Altorki, N. K. et al. Neoadjuvant durvalumab with or without stereotactic body radiotherapy in patients with early-stage non-small-cell lung cancer: a single-centre, randomised phase 2 trial. Lancet Oncol. 22, 824–835 (2021).
Tong, B. C. et al. Perioperative outcomes of pulmonary resection after neoadjuvant pembrolizumab in patients with non-small cell lung cancer. J. Thorac. Cardiovasc. Surg. 163, 427–436 (2022).
Chaft, J. E. et al. Neoadjuvant atezolizumab for resectable non-small cell lung cancer: an open-label, single-arm phase II trial. Nat. Med. 28, 2155–2161 (2022).
Wislez, M. et al. Neoadjuvant durvalumab for resectable non-small-cell lung cancer (NSCLC): results from a multicenter study (IFCT-1601 IONESCO). J. Immunother. Cancer 10, e005636 (2022).
Cascone, T. et al. Neoadjuvant durvalumab alone or combined with novel immuno-oncology agents in resectable lung cancer: the phase II NeoCOAST platform trial. Cancer Discov. 13, 2394–2411 (2023).
Awad, M. M. et al. 1261O Neoadjuvant nivolumab (N) + ipilimumab (I) vs chemotherapy (C) in the phase III CheckMate 816 trial. Ann. Oncol. 34, S731 (2023).
Schuler, M. et al. Neoadjuvant nivolumab with or without relatlimab in resectable non-small-cell lung cancer: a randomized phase 2 trial. Nat. Med. 30, 1602–1611 (2024).
Shu, C. A. et al. Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small-cell lung cancer: an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 21, 786–795 (2020).
Provencio, M. et al. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 21, 1413–1422 (2020).
Zhao, Z. R. et al. Phase 2 trial of neoadjuvant toripalimab with chemotherapy for resectable stage III non-small-cell lung cancer. Oncoimmunology 10, 1996000 (2021).
Provencio, M. et al. Perioperative nivolumab and chemotherapy in stage III non-small-cell lung cancer. N. Engl. J. Med. 389, 504–513 (2023).
Rothschild, S. I. et al. SAKK 16/14: Durvalumab in addition to neoadjuvant chemotherapy in patients with stage IIIA(N2) non-small-cell lung cancer—a multicenter single-arm phase II trial. J. Clin. Oncol. 39, 2872–2880 (2021).
Cascone, T. et al. Neoadjuvant chemotherapy plus nivolumab with or without ipilimumab in operable non-small cell lung cancer: the phase 2 platform NEOSTAR trial. Nat. Med. 29, 593–604 (2023).
Forde, P. M. et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N. Engl. J. Med. 386, 1973–1985 (2022).
Spicer, J. et al. Neoadjuvant nivolumab (NIVO) + chemotherapy (chemo) vs chemo in patients (pts) with resectable NSCLC: 4-year update from CheckMate 816. J. Clin. Oncol. 42, LBA8010 (2024).
US Food and Drug Administration. FDA approves neoadjuvant nivolumab and platinum-doublet chemotherapy for early-stage non-small cell lung cancer. FDA https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-neoadjuvant-nivolumab-and-platinum-doublet-chemotherapy-early-stage-non-small-cell-lung (2022).
European Medicines Agency. Opdivo. EMA https://www.ema.europa.eu/en/medicines/human/EPAR/opdivo (2023).
Wakelee, H. et al. Perioperative pembrolizumab for early-stage non-small-cell lung cancer. N. Engl. J. Med. 389, 491–503 (2023).
Heymach, J. V. et al. Perioperative durvalumab for resectable non-small-cell lung cancer. N. Engl. J. Med. 389, 1672–1684 (2023).
Cascone, T. et al. Perioperative nivolumab in resectable lung cancer. N. Engl. J. Med. 390, 1756–1769 (2024).
Spicer, J. D. et al. Neoadjuvant pembrolizumab plus chemotherapy followed by adjuvant pembrolizumab compared with neoadjuvant chemotherapy alone in patients with early-stage non-small-cell lung cancer (KEYNOTE-671): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 404, 1240–1252 (2024).
US Food and Drug Administration. FDA approves neoadjuvant/ adjuvant pembrolizumab for resectable non-small cell lung cancer. FDA https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-neoadjuvant-adjuvant-pembrolizumab-resectable-non-small-cell-lung-cancer (2023).
US Food and Drug Administration. FDA approves neoadjuvant/adjuvant durvalumab for resectable non-small cell lung cancer. FDA https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-neoadjuvantadjuvant-durvalumab-resectable-non-small-cell-lung-cancer (2024).
US Food and Drug Administration. FDA approves neoadjuvant/adjuvant nivolumab for resectable non-small cell lung cancer. FDA https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-neoadjuvantadjuvant-nivolumab-resectable-non-small-cell-lung-cancer (2024).
Heymach, J. V. et al. OA13.03 Perioperative durvalumab for resectable NSCLC (R-NSCLC): updated outcomes from the phase 3 AEGEAN trial. J. Thorac. Oncol. https://doi.org/10.1016/j.jtho.2024.09.069 (2024).
Provencio Pulla, M. et al. LBA50 Perioperative nivolumab (NIVO) v placebo (PBO) in patients (pts) with resectable NSCLC: clinical update from the phase III CheckMate 77T study. Ann. Oncol. 35, S1239–S1240 (2024).
André, T. et al. Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N. Engl. J. Med. 383, 2207–2218 (2020).
Chalabi, M. et al. Neoadjuvant immunotherapy in locally advanced mismatch repair-deficient colon cancer. N. Engl. J. Med. 390, 1949–1958 (2024).
Fokas, E. et al. Early efficacy end points in neoadjuvant rectal cancer trials: surrogacy revisited. J. Clin. Oncol. 42, 872–875 (2024).
Chalabi, M. et al. LBA24 Neoadjuvant immunotherapy in locally advanced MMR-deficient colon cancer: 3-year disease-free survival from NICHE-2. Ann. Oncol. 35, S1217–S1218 (2024).
Rousseau, B., White, J. R., Cercek, A. & Diaz, L. A. Jr. The duration of immunotherapy for mismatch repair-deficient cancers. N. Engl. J. Med. 392, 824–826 (2025).
Shiu, K.-K. et al. NEOPRISM-CRC: neoadjuvant pembrolizumab stratified to tumour mutation burden for high risk stage 2 or stage 3 deficient-MMR/MSI-high colorectal cancer. J. Clin. Oncol. 42, LBA3504 (2024).
Verschoor, Y. L. et al. LBA31 Neoadjuvant nivolumab plus relatlimab (anti-LAG3) in locally advanced MMR-deficient colon cancers: the NICHE-3 study. Ann. Oncol. 34, S1270 (2023).
de Gooyer, P. G. M. et al. Neoadjuvant nivolumab and relatlimab in locally advanced MMR-deficient colon cancer: a phase 2 trial. Nat. Med. 30, 3284–3290 (2024).
de la Fouchardiere, C. et al. 504O IMHOTEP phase II trial of neoadjuvant pembrolizumab in dMMR/MSI tumors: results of the colorectal cancer cohort. Ann. Oncol. 35, S429 (2024).
Hu, H. et al. Neoadjuvant PD-1 blockade with toripalimab, with or without celecoxib, in mismatch repair-deficient or microsatellite instability-high, locally advanced, colorectal cancer (PICC): a single-centre, parallel-group, non-comparative, randomised, phase 2 trial. Lancet Gastroenterol. Hepatol. 7, 38–48 (2022).
Xu, R. et al. Neoadjuvant treatment of IBI310 (anti-CTLA-4 antibody) plus sintilimab (anti-PD-1 antibody) in patients with microsatellite instability-high/mismatch repair-deficient colorectal cancer: results from a randomized, open-labeled, phase Ib study. J. Clin. Oncol. 42, 3505 (2024).
Cercek, A. et al. PD-1 blockade in mismatch repair-deficient, locally advanced rectal cancer. N. Engl. J. Med. 386, 2363–2376 (2022).
Cercek, A. et al. Durable complete responses to PD-1 blockade alone in mismatch repair deficient locally advanced rectal cancer. J. Clin. Oncol. 42, LBA3512 (2024).
Chalabi, M. et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat. Med. 26, 566–576 (2020).
Hissong, E. et al. Neoadjuvant botensilimab (BOT) plus balstilimab (BAL) in resectable mismatch repair proficient (pMMR) and deficient (dMMR) colorectal cancer (CRC): NEST clinical trial update. J. Clin. Oncol. 43, 207 (2025).
Ghelardi, F. et al. Preoperative botensilimab (BOT) with or without balstilimab (BAL) for patients with resectable locally advanced pMMR or dMMR colon cancer: results from the UNICORN trial by GONO. J. Clin. Oncol. 43, 158 (2025).
Sobral-Leite, M. et al. Assessment of PD-L1 expression across breast cancer molecular subtypes, in relation to mutation rate, BRCA1-like status, tumor-infiltrating immune cells and survival. Oncoimmunology 7, e1509820 (2018).
Schmid, P. et al. Pembrolizumab for early triple-negative breast cancer. N. Engl. J. Med. 382, 810–821 (2020).
Schmid, P. et al. Event-free survival with pembrolizumab in early triple-negative breast cancer. N. Engl. J. Med. 386, 556–567 (2022).
Schmid, P. et al. Overall survival with pembrolizumab in early-stage triple-negative breast cancer. N. Engl. J. Med. 391, 1981–1991 (2024).
Pusztai, L. et al. Event-free survival by residual cancer burden with pembrolizumab in early-stage TNBC: exploratory analysis from KEYNOTE-522. Ann. Oncol. 35, 429–436 (2024).
Schmid, P. et al. LBA4 Neoadjuvant pembrolizumab or placebo plus chemotherapy followed by adjuvant pembrolizumab or placebo for high-risk early-stage TNBC: overall survival results from the phase III KEYNOTE-522 study. Ann. Oncol. 35, S1204–S1205 (2024).
Mittendorf, E. A. et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. Lancet 396, 1090–1100 (2020).
Loibl, S. et al. Neoadjuvant durvalumab improves survival in early triple-negative breast cancer independent of pathological complete response. Ann. Oncol. 33, 1149–1158 (2022).
Sharma, P. et al. Clinical and biomarker findings of neoadjuvant pembrolizumab and carboplatin plus docetaxel in triple-negative breast cancer: NeoPACT phase 2 clinical trial. JAMA Oncol. 10, 227–235 (2024).
Ignatiadis, M. et al. Abstract GS01-03: Adding atezolizumab to adjuvant chemotherapy for stage II and III triple-negative breast cancer is unlikely to improve efficacy: interim analysis of the ALEXANDRA/IMpassion030 phase 3 trial. Cancer Res. 84, GS01-03 (2024).
Conte, P. F. et al. A-BRAVE trial: a phase III randomized trial with avelumab in early triple-negative breast cancer with residual disease after neoadjuvant chemotherapy or at high risk after primary surgery and adjuvant chemotherapy. J. Clin. Oncol. 42, LBA500 (2024).
Nederlof, I. et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in early-stage triple-negative breast cancer: a phase 2 adaptive trial. Nat. Med. 30, 3223–3235 (2024).
Nederlof, I. et al. LBA11 Neoadjuvant nivolumab/relatlimab or nivolumab/ipilimumab in triple negative breast cancer with high tumor-infiltrating lymphocytes (TILs). Ann. Oncol. 35, S1206 (2024).
Huober, J. et al. Atezolizumab with neoadjuvant anti-human epidermal growth factor receptor 2 therapy and chemotherapy in human epidermal growth factor receptor 2-positive early breast cancer: primary results of the randomized phase III IMpassion050 trial. J. Clin. Oncol. 40, 2946–2956 (2022).
Rinnerthaler, G. et al. 123O Randomized phase II trial of neoadjuvant atezolizumab in combination with dual HER2 blockade plus epirubicin in early HER2-positive breast cancer (ABCSG-52/ATHENE). ESMO Open 8, 101462 (2023).
Voorwerk, L. et al. Immune landscape of breast tumors with low and intermediate estrogen receptor expression. NPJ Breast Cancer 9, 39 (2023).
Nanda, R. et al. Effect of pembrolizumab plus neoadjuvant chemotherapy on pathologic complete response in women with early-stage breast cancer: an analysis of the ongoing phase 2 adaptively randomized I-SPY2 trial. JAMA Oncol. 6, 676–684 (2020).
Pusztai, L. et al. Durvalumab with olaparib and paclitaxel for high-risk HER2-negative stage II/III breast cancer: results from the adaptively randomized I-SPY2 trial. Cancer Cell 39, 989–998 (2021).
Loi, S. et al. LBA20 A randomized, double-blind trial of nivolumab (NIVO) vs placebo (PBO) with neoadjuvant chemotherapy (NACT) followed by adjuvant endocrine therapy (ET) ± NIVO in patients (pts) with high-risk, ER+ HER2− primary breast cancer (BC). Ann. Oncol. 34, S1259–S1260 (2023).
Loi, S. et al. Neoadjuvant nivolumab and chemotherapy in early estrogen receptor-positive breast cancer: a randomized phase 3 trial. Nat. Med. 31, 433–441 (2025).
Cardoso, F. et al. LBA21 KEYNOTE-756: phase III study of neoadjuvant pembrolizumab (pembro) or placebo (pbo) + chemotherapy (chemo), followed by adjuvant pembro or pbo + endocrine therapy (ET) for early-stage high-risk ER+/HER2− breast cancer. Ann. Oncol. 34, S1260–S1261 (2023).
Cardoso, F. et al. Neoadjuvant pembrolizumab or placebo + chemotherapy, followed by adjuvant pembrolizumab or placebo plus endocrine therapy for early-stage high-risk ER+/HER2− breast cancer: results from the phase 3 KEYNOTE-756 study. Eur. J. Cancer 200, 113608 (2024).
van der Heijden, M. S. et al. Nivolumab plus gemcitabine–cisplatin in advanced urothelial carcinoma. N. Engl. J. Med. 389, 1778–1789 (2023).
Powles, T. et al. Enfortumab vedotin and pembrolizumab in untreated advanced urothelial cancer. N. Engl. J. Med. 390, 875–888 (2024).
Apolo, A. et al. AMBASSADOR Alliance A031501: phase III randomized adjuvant study of pembrolizumab in muscle-invasive and locally advanced urothelial carcinoma (MIUC) vs observation. J. Clin. Oncol. 42, LBA531 (2024).
Bellmunt, J. et al. Adjuvant atezolizumab versus observation in muscle-invasive urothelial carcinoma (IMvigor010): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 22, 525–537 (2021).
Necchi, A. et al. Updated results of PURE-01 with preliminary activity of neoadjuvant pembrolizumab in patients with muscle-invasive bladder carcinoma with variant histologies. Eur. Urol. 77, 439–446 (2020).
Powles, T. et al. Clinical efficacy and biomarker analysis of neoadjuvant atezolizumab in operable urothelial carcinoma in the ABACUS trial. Nat. Med. 25, 1706–1714 (2019).
Necchi, A. et al. Pembrolizumab as neoadjuvant therapy before radical cystectomy in patients with muscle-invasive urothelial bladder carcinoma (PURE-01): an open-label, single-arm, phase II study. J. Clin. Oncol. 36, 3353–3360 (2018).
Carthon, B. C. et al. Preoperative CTLA-4 blockade: tolerability and immune monitoring in the setting of a presurgical clinical trial. Clin. Cancer Res. 16, 2861–2871 (2010).
van Dijk, N. et al. Preoperative ipilimumab plus nivolumab in locoregionally advanced urothelial cancer: the NABUCCO trial. Nat. Med. 26, 1839–1844 (2020).
Szabados, B. et al. Final results of neoadjuvant atezolizumab in cisplatin-ineligible patients with muscle-invasive urothelial cancer of the bladder. Eur. Urol. 82, 212–222 (2022).
Galsky, M. D. et al. Gemcitabine and cisplatin plus nivolumab as organ-sparing treatment for muscle-invasive bladder cancer: a phase 2 trial. Nat. Med. 29, 2825–2834 (2023).
Stockem, C. F. et al. Induction therapy with ipilimumab and nivolumab followed by consolidative chemoradiation as organ-sparing treatment in urothelial bladder cancer: study protocol of the INDIBLADE trial. Front. Oncol. 13, 1246603 (2023).
Powles, T. et al. Perioperative durvalumab with neoadjuvant chemotherapy in operable bladder cancer. N. Engl. J. Med. 391, 1773–1786 (2024).
Houédé, N. et al. Safety and efficacy of neoadjuvant durvalumab plus gemcitabine/cisplatin or carboplatin in patients with operable high-risk upper tract urothelial carcinoma: the iNDUCT-GETUG V08 trial. J. Clin. Oncol. 43, 1578–1586 (2025).
Choueiri, T. K. et al. Adjuvant pembrolizumab after nephrectomy in renal-cell carcinoma. N. Engl. J. Med. 385, 683–694 (2021).
Allaf, M. E. et al. Perioperative nivolumab versus observation in patients with renal cell carcinoma undergoing nephrectomy (PROSPER ECOG-ACRIN EA8143): an open-label, randomised, phase 3 study. Lancet Oncol. 25, 1038–1052 (2024).
Ornstein, M. et al. A phase Ib trial of neoadjuvant/adjuvant durvalumab ± tremelimumab in locally advanced renal cell carcinoma (RCC). J. Clin. Oncol. 38, 5021 (2020).
Li, J., Luo, Z. & Jiang, S. Advancements in neoadjuvant immune checkpoint inhibitor therapy for locally advanced head and neck squamous carcinoma: a narrative review. Int. Immunopharmacol. 134, 112200 (2024).
Wise-Draper, T. M. et al. Phase II clinical trial of neoadjuvant and adjuvant pembrolizumab in resectable local-regionally advanced head and neck squamous cell carcinoma. Clin. Cancer Res. 28, 1345–1352 (2022).
Ferris, R. L. et al. Neoadjuvant nivolumab alone or in combination with relatlimab or ipilimumab in resectable head and neck squamous cell carcinoma (HNSCC). J. Clin. Oncol. 41, 6018 (2023).
Rosenberg, A. J. et al. Neoadjuvant nivolumab plus chemotherapy followed by response-stratified chemoradiation therapy in HPV-negative head and neck cancer: the DEPEND phase 2 nonrandomized clinical trial. JAMA Oncol. 6, e250081 (2025).
Shitara, K. et al. Neoadjuvant and adjuvant pembrolizumab plus chemotherapy in locally advanced gastric or gastro-oesophageal cancer (KEYNOTE-585): an interim analysis of the multicentre, double-blind, randomised phase 3 study. Lancet Oncol. 25, 212–224 (2024).
Janjigian, Y. et al. LBA73 Pathological complete response (pCR) to durvalumab plus 5-fluorouracil, leucovorin, oxaliplatin and docetaxel (FLOT) in resectable gastric and gastroesophageal junction cancer (GC/GEJC): interim results of the global, phase III MATTERHORN study. Ann. Oncol. 34, S1315–S1316 (2023).
Al-Batran, S. et al. Surgical and pathological outcome, and pathological regression, in patients receiving perioperative atezolizumab in combination with FLOT chemotherapy versus FLOT alone for resectable esophagogastric adenocarcinoma: interim results from DANTE, a randomized, multicenter, phase IIb trial of the FLOT-AIO German Gastric Cancer Group and Swiss SAKK. J. Clin. Oncol. 40, 4003 (2024).
Lorenzen, S. et al. Perioperative atezolizumab plus fluorouracil, leucovorin, oxaliplatin, and docetaxel for resectable esophagogastric cancer: interim results from the randomized, multicenter, phase II/III DANTE/IKF-s633 trial. J. Clin. Oncol. 42, 410–420 (2024).
Verschoor, Y. L. et al. Neoadjuvant atezolizumab plus chemotherapy in gastric and gastroesophageal junction adenocarcinoma: the phase 2 PANDA trial. Nat. Med. 30, 519–530 (2024).
André, T. et al. Neoadjuvant nivolumab plus ipilimumab and adjuvant nivolumab in localized deficient mismatch repair/microsatellite instability-high gastric or esophagogastric junction adenocarcinoma: the GERCOR NEONIPIGA phase II study. J. Clin. Oncol. 41, 255–265 (2023).
Raimondi, A. et al. Tremelimumab and durvalumab as neoadjuvant or non-operative management strategy of patients with microsatellite instability-high resectable gastric or gastroesophageal junction adenocarcinoma: the INFINITY study by GONO. Ann. Oncol. 36, 285–296 (2024).
Wilde, D. C., Glaun, M. E., Wong, M. K. & Gross, N. D. Neoadjuvant approaches to non-melanoma skin cancer. Cancers 15, 5494 (2023).
Jones, G. et al. Abstract 7518: Neoadjuvant–adjuvant pembrolizumab in resectable advanced basal cell carcinoma of the head and neck: an open-label, single-arm, phase 1b trial. Cancer Res. 84, 7518 (2024).
Que, S. K. T., Zwald, F. O. & Schmults, C. D. Cutaneous squamous cell carcinoma: management of advanced and high-stage tumors. J. Am. Acad. Dermatol. 78, 249–261 (2018).
Gross, N. D. et al. Neoadjuvant cemiplimab for stage II to IV cutaneous squamous-cell carcinoma. N. Engl. J. Med. 387, 1557–1568 (2022).
Gross, N. D. et al. Neoadjuvant cemiplimab and surgery for stage II–IV cutaneous squamous-cell carcinoma: follow-up and survival outcomes of a single-arm, multicentre, phase 2 study. Lancet Oncol. 24, 1196–1205 (2023).
Ascierto, P. et al. NEO-CESQ study: neoadjuvant plus adjuvant treatment with cemiplimab in surgically resectable, high risk stage III/IV (M0) cutaneous squamous cell carcinoma. J. Clin. Oncol. 41, 9576 (2023).
Zuur, C. L. et al. Towards organ preservation and cure via 2 infusions of immunotherapy only, in patients normally undergoing extensive and mutilating curative surgery for cutaneous squamous cell carcinoma: an investigator-initiated randomized phase II trial—the MATISSE trial. J. Clin. Oncol. 41, 9507 (2023).
Topalian, S. L. et al. Neoadjuvant nivolumab for patients with resectable Merkel cell carcinoma in the CheckMate 358 trial. J. Clin. Oncol. 38, 2476–2487 (2020).
Cloughesy, T. F. et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat. Med. 25, 477–486 (2019).
Long, G. V. et al. Neoadjuvant triplet immune checkpoint blockade in newly diagnosed glioblastoma. Nat. Med. https://doi.org/10.1038/s41591-025-03512-1 (2025).
Dummer, R. et al. Neoadjuvant anti-PD-1 alone or in combination with anti-TIGIT or an oncolytic virus in resectable stage IIIB–D melanoma: a phase 1/2 trial. Nat. Med. 31, 144–151 (2025).
Wolf, Y., Anderson, A. C. & Kuchroo, V. K. TIM3 comes of age as an inhibitory receptor. Nat. Rev. Immunol. 20, 173–185 (2020).
Creelan, B. C. & Antonia, S. J. The NKG2A immune checkpoint — a new direction in cancer immunotherapy. Nat. Rev. Clin. Oncol. 16, 277–278 (2019).
Barlesi, F. et al. Phase 3 study of durvalumab combined with oleclumab or monalizumab in patients with unresectable stage III NSCLC (PACIFIC-9). J. Clin. Oncol. 41, TPS8610 (2023).
Cascone, T. et al. PL02.07 Neocoast-2: efficacy and safety of neoadjuvant durvalumab (D) + novel anticancer agents + CT and adjuvant D ± novel agents in resectable NSCLC. J. Thorac. Oncol. https://doi.org/10.1016/j.jtho.2024.09.013 (2024).
Weber, J. S. et al. Individualised neoantigen therapy mRNA-4157 (V940) plus pembrolizumab versus pembrolizumab monotherapy in resected melanoma (KEYNOTE-942): a randomised, phase 2b study. Lancet 403, 632–644 (2024).
Davar, D. et al. Neoadjuvant vidutolimod and nivolumab in high-risk resectable melanoma: a prospective phase II trial. Cancer Cell 42, 1898–1918 (2024).
Thomas, R. J. & Bartee, E. The use of oncolytic virotherapy in the neoadjuvant setting. J. Immunother. Cancer 10, e004462 (2022).
Andtbacka, R. H. I. et al. Final analyses of OPTiM: a randomized phase III trial of talimogene laherparepvec versus granulocyte–macrophage colony-stimulating factor in unresectable stage III–IV melanoma. J. Immunother. Cancer 7, 145 (2019).
Soliman, H. et al. Oncolytic T-VEC virotherapy plus neoadjuvant chemotherapy in nonmetastatic triple-negative breast cancer: a phase 2 trial. Nat. Med. 29, 450–457 (2023).
Nguyen, V. P. et al. A pilot study of neoadjuvant nivolumab, ipilimumab, and intralesional oncolytic virotherapy for HER2-negative breast cancer. Cancer Res. Commun. 3, 1628–1637 (2023).
Bastien, E. et al. 319TiP NeoBREASTIM: a phase II study of atezolizumab plus RP1 oncolytic immunotherapy in the neoadjuvant setting of triple-negative breast cancer (TNBC). Ann. Oncol. 35, S347–S348 (2024).
Cortés, J. et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N. Engl. J. Med. 386, 1143–1154 (2022).
Shitara, K. et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N. Engl. J. Med. 382, 2419–2430 (2020).
Smit, E. F. et al. Trastuzumab deruxtecan in patients with metastatic non-small-cell lung cancer (DESTINY-Lung01): primary results of the HER2-overexpressing cohorts from a single-arm, phase 2 trial. Lancet Oncol. 25, 439–454 (2024).
Bardia, A. et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. N. Engl. J. Med. 384, 1529–1541 (2021).
Jhaveri, K. et al. LBA2 Datopotamab deruxtecan (Dato-DXd) vs chemotherapy (CT) in pretreated, inoperable/metastatic HR+/HER2− breast cancer (BC): additional safety analysis from TROPION-Breast01. ESMO Open 9, 103479 (2024).
Ahn, M. J. et al. LBA12 Datopotamab deruxtecan (Dato-DXd) vs docetaxel in previously treated advanced/metastatic (adv/met) non-small cell lung cancer (NSCLC): results of the randomized phase III study TROPION-Lung01. Ann. Oncol. 34, S1305–S1306 (2023).
Shatsky, R. A. et al. Datopotamab–deruxtecan plus durvalumab in early-stage breast cancer: the sequential multiple assignment randomized I-SPY2.2 phase 2 trial. Nat. Med. 30, 3737–3747 (2024).
Bex, A. et al. Efficacy, safety, and biomarker analysis of neoadjuvant avelumab/axitinib in patients (pts) with localized renal cell carcinoma (RCC) who are at high risk of relapse after nephrectomy (NeoAvAx). J. Clin. Oncol. 40, 289 (2022).
Long, G. V. et al. 793P NeoPeLe: a phase II trial of neoadjuvant (NAT) pembrolizumab (Pembro) combined with lenvatinib (Lenva) in resectable stage III melanoma. Ann. Oncol. 33, S906–S907 (2022).
Hendriks, L. E. et al. Oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 34, 339–357 (2023).
Strohbehn, G. W. & Gyawali, B. ‘Contribution of component’ and the perioperative immune-checkpoint inhibitor precedent. Nat. Rev. Clin. Oncol. 21, 249–250 (2024).
Kok, M. et al. Academic uphill battle to personalize treatment for patients with stage II/III triple-negative breast cancer. J. Clin. Oncol. 42, 3523–3529 (2024).
Jones, D. R. et al. OA01.03 Association of pathologic regression with EFS in the KEYNOTE-671 study of perioperative pembrolizumab for early-stage NSCLC. J. Thorac. Oncol. https://doi.org/10.1016/j.jtho.2024.09.022 (2024).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
G.A. declares honoraria from Novartis, consulting fees paid to the institute by Novartis, research funding to the institute from Novartis, Stichting tegen Kanker, Kom op tegen Kanker, research funding from ESMO and FWO; and travel, accommodations and expenses from Gilead Sciences and Pierre Fabre. T.C. reports speaker fees and/or honoraria (including travel and/or meeting expenses) from ASCO Post, AstraZeneca, Bio Ascend, Bristol Myers Squibb, Clinical Care Options, IDEOlogy Health, the Medical Educator Consortium, Medscape, OncLive, PEAK Medicals, PeerView, Physicians’ Education Resource and Targeted Oncology; advisory role and/or consulting fees (including travel and/or meeting expenses) from AstraZeneca, Bristol Myers Squibb, Daiichi Sankyo, Genentech, Merck, oNKo-innate, Pfizer, RAPT Therapeutics and Regeneron; and institutional research funding from AstraZeneca and Bristol Myers Squibb. M.S.v.d.H. declares research support from Bristol Meyers Squibb, AstraZeneca, Roche, 4SC and Merch Sharp & Dohme; and consultancy fees from Bristol Meyers Squibb, Merch Sharp & Dohme, Roche, AstraZeneca, Seagen, Pfizer, Janssen and Daiichi Sankyo. All grants were paid to the institute. C.U.B. has received research grants from Novartis, BMS and NanoString, is a paid advisory board member for BMS, MSD, Roche, Novartis, GlaxoSmithKline, AstraZeneca, Pfizer, Lilly, Genmab and Pierre Fabre and holds ownership interest in Uniti Card, Neon Therapeutics and Forty Seven, all outside this submitted work. M.K. reports research grants from AZ–Daiichi, BMS and Roche, speaker fees from AZ–Daiichi and Gilead, compensation for advisory work from Alderaan Biotechnology, AZ–Daiichi, BioNTech, BMS, Domain Therapeutics, Gilead, MSD, Novartis and Roche and nonfinancial support from Natera. All grants were paid to the institute. M.C. is advisor to Bristol Myers Squibb, Merck Sharp & Dohme, Agenus and Roche–Genentech and has received research grants unrelated to this study from Agenus, Merck Sharp & Dohme and Roche–Genentech. All grants were paid to the institute.
Peer review
Peer review information
Nature Cancer thanks Matthew Block, Ignacio Melero, Michael Postow and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Awada, G., Cascone, T., van der Heijden, M.S. et al. The rapidly evolving paradigm of neoadjuvant immunotherapy across cancer types. Nat Cancer 6, 967–987 (2025). https://doi.org/10.1038/s43018-025-00990-7
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s43018-025-00990-7