Abstract
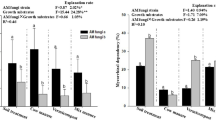
While microplastic impacts on aquatic and agricultural systems are well-documented, their impacts on forest ecosystems remain poorly understood. We assessed how microplastic addition affects rhizosphere soil properties and fine-root traits for ectomycorrhizal (ECM) and arbuscular mycorrhizal (AM) associations in a mixed temperate forest. In ECM-associated soils, microplastics increased nitrogen availability and nitrate reductase activity but decreased phosphorus and phosphatase activity; AM-associated soils showed the opposite pattern. Morphologically, ECM roots exhibited reduced branching but increased hyphal density and colonization. Conversely, AM roots displayed increased specific root length and tip density but decreased cortical thickness and tissue density. These divergent, mycorrhizal-specific responses suggest that increasing microplastic pollution may fundamentally alter nutrient cycling and species composition dynamics in temperate forests.
Similar content being viewed by others
Data availability
The data utilized in this study are accessible from https://doi.org/10.5281/zenodo.17538924.
References
Bergmann, M. et al. White and wonderful? Microplastics prevail in snow from the Alps to the Arctic. Sci. Adv. 5, eaax1157 (2019).
Rillig, M. C. Microplastic disguising as soil carbon storage. Environ. Sci. Technol. 52, 6079–6080 (2018).
Liese, B. et al. Uptake of microplastics and impacts on plant traits of savoy cabbage. Ecotoxicol. Environ. Saf. 272, 116086 (2024).
Ma, R. et al. Microplastics affect C, N, and P cycling in natural environments: highlighting the driver of soil hydraulic properties. J. Hazard. Mater. 459, 132326 (2023).
Richard, C. T. et al. Lost at sea: where is all the plastic? Science 304, 838 (2004).
Li, H. et al. Single and composite damage mechanisms of soil polyethylene/polyvinyl chloride microplastics to the photosynthetic performance of soybean (Glycine max [L.] merr.). Front. Plant Sci. 13, 1100291 (2023).
Li, R. et al. Visual tracking of label-free microplastics in wheat seedlings and their effects on crop growth and physiology. J. Hazard. Mater. 456, 131675 (2023).
Weber, C. J., Rillig, M. C. & Bigalke, M. Mind the gap: forest soils as a hidden hub for global micro- and nanoplastic pollution. Microplastics Nanoplastics 3, 19 (2023).
Green, J. K. & Keena, T. F. The limits of forest carbon sequestration. Science 376, 692–693 (2022).
Cayuela, C., Levia, D. F., Latron, J. & Llorens, P. Particulate matter fluxes in a mediterranean mountain forest: interspecific differences between throughfall and stemflow in oak and pinestands. J. Geophys. Res. Atmos. 124, 5106–5116 (2019).
Wang, W. et al. Responses of lettuce (Lactuca sativa L.) growth and soil properties to conventional non-biodegradable and new biodegradable microplastics. Environ. Pollut. 341, 122897 (2024).
Ranauda, M. A. et al. From the rhizosphere to plant fitness: implications of microplastics soil pollution. Environ. Exp. Bot. 226, 105874 (2024).
de Souza Machado, A. A., Kloas, W., Zarfl, C., Hempel, S. & Rillig, M. C. Microplastics as an emerging threat to terrestrial ecosystems. Glob. Change Biol. 24, 1405–1416 (2018).
Guerrero-Ramírez, N. et al. Global root traits (GRooT) database. Glob. Ecol. Biogeogr. 30, 25–37 (2020).
Guo, W. et al. Linking fine-root diameter across root orders with climatic, biological and edaphic factors in the northern hemisphere. Oikos 2024, e10763 (2024).
Xu, H. et al. Effects of microplastics concentration on plant root traits and biomass: experiment and meta-analysis. Ecotoxicol. Environ. Saf. 285, 117038 (2024).
Sun, H., Lei, C., Xu, J. & Li, R. Foliar uptake and leaf-to-root translocation of nanoplastics with different coating charge in maize plants. J. Hazard. Mater. 416, 125854 (2021).
Rozman, U. et al. An extensive characterization of various environmentally relevant microplastics–material properties, leaching and ecotoxicity testing. Sci. Total Environ. 773, 145576 (2021).
Lehmann, A. et al. Microplastic fiber and drought effects on plants and soil are only slightly modified by arbuscular mycorrhizal fungi. Soil Ecol. Lett. 4, 32–44 (2020).
Chen, H. et al. Arbuscular mycorrhizal fungi can inhibit the allocation of microplastics from crop roots to aboveground edible parts. J. Agric. Food Chem. 71, 18323–18332 (2023).
Urbina, M. A., Correa, F., Aburto, F. & Ferrio, J. P. Adsorption of polyethylene microbeads and physiological effects on hydroponic maize. Sci. Total Environ. 741, 140216 (2020).
Spanò, C. et al. Polystyrene nanoplastics affect seed germination, cell biology and physiology of rice seedlings in-short term treatments: evidence of their internalization and translocation. Plant Physiol. Biochem. 172, 158–166 (2022).
Chen, W. et al. Root morphology and mycorrhizal symbioses together shape nutrient foraging strategies of temperate trees. Proc. Natl. Acad. Sci. USA 113, 8741–8746 (2016).
Averill, C., Bhatnagar, J. M., Dietze, M. C., Pearse, W. D. & Kivlin, S. N. Global imprint of mycorrhizal fungi on whole-plant nutrient economics. Proc. Natl. Acad. Sci. USA 116, 23163–23168 (2019).
van der Heijden, M. G. A., Martin, F. M., Selosse, M. A. & Sanders, I. R. Mycorrhizal ecology and evolution: the past, the present, and the future. N. Phytol. 205, 1406–1423 (2015).
Rillig, M. C., Lehmann, A., Mounts, I. R. & Bock, B. M. Concurrent common fungal networks formed by different guilds of fungi. N. Phytol. 246, 33–38 (2025).
Choreño-Parra, E. M. & Treseder, K. K. Mycorrhizal fungi modify decomposition: a meta-analysis. N. Phytol. 242, 2763–2774 (2024).
Mayer, M. et al. Soil fertility determines whether ectomycorrhizal fungi accelerate or decelerate decomposition in a temperate forest. N. Phytol. 239, 325–339 (2023).
Leifheit, E. F., Lehmann, A. & Rillig, M. C. Potential effects of microplastic on arbuscular mycorrhizal fungi. Front. Plant Sci. 12, 626709 (2021).
Kanold, E., Buchanan, S. W., Dunfield, K. & Antunes, P. M. Microplastic additions modulate intraspecific variability in root traits and mycorrhizal responses across root-life history strategies. Funct. Ecol. 38, 2369–2377 (2024).
de Souza Machado, A. A. et al. Microplastics can change soil properties and affect plant performance. Environ. Sci. Technol. 53, 6044–6052 (2019).
Read, D. J. Mycorrhizas in ecosystems. Experientia 47, 376–391 (1991).
Chari, N. R., Muratore, T. J. & Taylor, B. N. Long-term soil warming drives different belowground responses in arbuscular mycorrhizal and ectomycorrhizal trees. Glob. Change Biol. 30, 1–11 (2024).
Pregitzer, K. S. et al. Fine root architecture of nine North American trees. Ecol. Monogr. 72, 293–309 (2002).
Terrer, C., Vicca, S., Hungate, B. A., Phillips, R. P. & Prentice, I. C. Mycorrhizal association as a primary control of the CO2 fertilization effect. Science 353, 72–74 (2016).
Austen, K., MacLean, J., Balanzategui, D. & Hölker, F. Microplastic inclusion in birch tree roots. Sci. Total Environ. 808, 152085 (2022).
Midgley, M. G. & Phillips, R. P. Mycorrhizal associations of dominant trees influence nitrate leaching responses to N deposition. Biogeochemistry 117, 241–253 (2013).
Kuyper, T. W. & Jansa, J. Arbuscular mycorrhiza: advances and retreats in our understanding of the ecological functioning of the mother of all root symbioses. Plant Soil 489, 41–88 (2023).
Wen, H. et al. Diverse and high pollution of microplastics in seasonal snow across northeastern China. Sci. Total Environ. 907, 167923 (2024).
Wang, Q. et al. Effects of microplastics and carbon nanotubes on soil geochemical properties and bacterial communities. J. Hazard. Mater. 433, 128826 (2022).
Dijkstra, F. A., Carrillo, Y., Pendall, E. & Morgan, J. A. Rhizosphere priming: a nutrient perspective. Front. Microbiol. 4, 216 (2013).
Phillips, R. P. & Fahey, T. J. The influence of soil fertility on rhizosphere effects in northern hardwood forest soils. Soil Sci. Soc. Am. J. 72, 453–461 (2008).
Huang, S. et al. Polyethylene and polyvinyl chloride microplastics promote soil nitrification and alter the composition of key nitrogen functional bacterial groups. J. Hazard. Mater. 453, 131391 (2023).
Zhou, J., Xu, H., Xiang, Y. & Wu, J. Effects of microplastics pollution on plant and soil phosphorus: a meta-analysis. J. Hazard. Mater. 461, 132705 (2024).
Gao, B., Yao, H., Li, Y. & Zhu, Y. Microplastic addition alters the microbial community structure and stimulates soil carbon dioxide emissions in vegetable-growing soil. Environ. Toxicol. Chem. 40, 352–365 (2021).
Rillig, M. C., Lehmann, A., de Souza Machado, A. A. & Yang, G. Microplastic effects on plants. N. Phytol. 223, 1066–1070 (2019).
Qin, W., Hu, C. & Oenema, O. Soil mulching significantly enhances yields and water and nitrogen use efficiencies of maize and wheat: a meta-analysis. Sci. Rep. 5, 16210 (2015).
Sajjad, M. et al. Microplastics in the soil environment: a critical review. Environ. Technol. Innov. 27, 102408 (2022).
Zhang, J. et al. Effects of plastic residues and microplastics on soil ecosystems: a global meta-analysis. J. Hazard. Mater. 435, 129065 (2022).
Wang, F., Wang, Q., Adams, C. A., Sun, Y. & Zhang, S. Effects of microplastics on soil properties: current knowledge and future perspectives. J. Hazard. Mater. 424, 127531 (2022).
Tong, Y. et al. Microplastics affect activity and spatial distribution of C, N, and P hydrolases in rice rhizosphere. Soil Ecol. Lett. 5, 220138 (2022).
Lozano, Y. M. et al. Effects of microplastics and drought on soil ecosystem functions and multifunctionality. J. Appl. Ecol. 58, 988–996 (2021).
Zhou, Y. et al. Polystyrene microplastic pollution induces species-specific shifts in root traits and rhizosphere conditions in a temperate forest. J. Hazard. Mater. 495, 139032 (2025).
Fei, Y. et al. Response of soil enzyme activities and bacterial communities to the accumulation of microplastics in an acid cropped soil. Sci. Total Environ. 707, 135634 (2020).
Yan, Y. et al. Effect of polyvinyl chloride microplastics on bacterial community and nutrient status in two agricultural soils. Bull. Environ. Contam. Toxicol. 107, 602–609 (2021).
Ceccanti, C. et al. Polyethylene microplastics alter root functionality and affect strawberry plant physiology and fruit quality traits. J. Hazard. Mater. 470, 134164 (2024).
Bosker, T., Bouwman, L. J., Brun, N. R., Behrens, P. & Vijver, M. G. Microplastics accumulate on pores in seed capsule and delay germination and root growth of the terrestrial vascular plant Lepidium sativum. Chemosphere 226, 774–781 (2019).
Liu, Y. et al. Microplastics reduce nitrogen uptake in peanut plants by damaging root cells and impairing soil nitrogen cycling. J. Hazard. Mater. 443, 130384 (2023).
Lian, P., Xu, L., Yang, L., Yue, K. & Peñuelas, J. Divergent soil P accrual in ectomycorrhizal and arbuscular mycorrhizal trees: insights from a common garden experiment in subtropical China. Front. Plant Sci. 15, 1333505 (2024).
Phillips, R. P., Brzostek, E. & Midgley, M. G. The mycorrhizal-associated nutrient economy: a new framework for predicting carbon-nutrient couplings in temperate forests. N. Phytol. 199, 41–51 (2013).
Zhu, X. et al. Extraradical hyphae exhibit more plastic nutrient-acquisition strategies than roots under nitrogen enrichment in ectomycorrhiza-dominated forests. Glob. Change Biol. 29, 4605–4619 (2023).
Finzi, A. C. et al. Rhizosphere processes are quantitatively important components of terrestrial carbon and nutrient cycles. Glob. Change Biol. 21, 2082–2094 (2015).
Fort, F. et al. Root traits are related to plant water-use among rangeland mediterranean species. Funct. Ecol. 31, 1700–1709 (2017).
Bergmann, J. et al. The fungal collaboration gradient dominates the root economics space in plants. Sci. Adv. 6, eaba3756 (2020).
Geng, P. & Jin, G. Fine root morphology and chemical responses to N addition depend on root function and soil depth in a Korean pine plantation in Northeast China. For. Ecol. Manag. 520, 120407 (2022).
Zantis, L. J. et al. Species-dependent responses of crop plants to polystyrene microplastics. Environ. Pollut. 335, 122243 (2023).
Zhang, Y. Q. et al. Microplastic abundance thresholds shape the growth of 18 wild plant species: the importance of soil pH. J. Plant Ecol. 18, rtaf066 (2025).
Jonathan, A. B. et al. Plant-soil feedbacks and mycorrhizal type influence temperate forest population dynamics. Science 355, 181–184 (2017).
Fahey, C., Bell, F. W. & Antunes, P. M. Effects of dual mycorrhizal inoculation on Pinus strobus seedlings are influenced by soil resource availability. Plant Soil 479, 607–620 (2022).
Tisserant, E. et al. Genome of an arbuscular mycorrhizal fungus provides insight into the oldest plant symbiosis. Proc. Natl. Acad. Sci. USA 110, 20117–20122 (2013).
Mathur, S., Tomar, R. S. & Jajoo, A. Arbuscular mycorrhizal fungi (AMF) protects photosynthetic apparatus of wheat under drought stress. Photosynth. Res. 139, 227–238 (2019).
Xie, K. et al. Plant nitrogen nutrition: the roles of arbuscular mycorrhizal fungi. J. Plant Physiol. 269, 153591 (2022).
Smith, S. E., Smith, F. A. & Jakobsen, I. Functional diversity in arbuscular mycorrhizal (AM) symbioses: the contribution of the mycorrhizal P uptake pathway is not correlated with mycorrhizal responses in growth or total P uptake. N. Phytol. 162, 511–524 (2004).
Jing, M., Shi, Z., Zhang, M., Zhang, M. & Wang, X. Nitrogen and phosphorus of plants associated with arbuscular and ectomycorrhizas are differentially influenced by drought. Plants 11, 2429 (2022).
Fu, W. et al. Community response of arbuscular mycorrhizal fungi to extreme drought in a cold-temperate grassland. N. Phytol. 234, 2003–2017 (2022).
Comas, L. H. & Eissenstat, D. M. Linking fine root traits to maximum potential growth rate among 11 mature temperate tree species. Funct. Ecol. 18, 388–397 (2004).
Fuller, S. & Gautam, A. A procedure for measuring microplastics using pressurized fluid extraction. Environ. Sci. Technol. 50, 5774–5780 (2016).
Huerta Lwanga, E. et al. Microplastics in the terrestrial ecosystem: implications for Lumbricus terrestris (Oligochaeta, Lumbricidae). Environ. Sci. Technol. 50, 2685–2691 (2016).
Zang, H. et al. Microplastics in the agroecosystem: are they an emerging threat to the plant-soil system? Soil Biol. Biochem. 148, 107926 (2020).
de Souza Machado, A. A. et al. Impacts of microplastics on the soil biophysical environment. Environ. Sci. Technol. 52, 9656–9665 (2018).
Lu, R. K. Analytical Methods for Soil and Agriculture Chemistry (ed Lu, R. K.) 312–314 (China Agricultural Science and Technology Press, 2000).
Gu, J., Xu, Y., Dong, X., Wang, H. & Wang, Z. Root diameter variations explained by anatomy and phylogeny of 50 tropical and temperate tree species. Tree Physiol. 34, 415–425 (2014).
McGonigle, T. P., Miller, M. H., Evans, D. G., Fairchild, G. L. & Swan, J. A. A new method which gives an objective measure of colonization of roots by vesicular—arbuscular mycorrhizal fungi. N. Phytol. 115, 495–501 (2006).
Mehlich, A. Mehlich 3 soil test extractant: a modification of Mehlich 2 extractant. Commun. Soil Sci. Plant Anal. 15, 1409–1416 (2008).
Eivazi, F. & Tabatabai, M. A. Glucosidases and galactosidases in soils. Soil Biol. Biochem. 5, 601–606 (1988).
Marx, M. C., Wood, M. & Jarvis, S. C. A microplate fluorimetric assay for the study of enzyme diversity in soils. Soil Biol. Biochem. 33, 1633–1640 (2001).
Brundrett, M. Based on a workshop organized in conjunction with the ninth North American conference on mycorrhizae. in Practical Methods in Mycorrhiza Research (University of Guelph, 1994).
Acknowledgements
This work was financially supported by the Natural Science Foundation of China (grant number 42171051).
Author information
Authors and Affiliations
Contributions
Yingtong Zhou: data curation and writing-original draft preparation. Ivano Brunner: writing-reviewing and editing. Ziping Liu: conceptualization and visualization. Wei Guo: software and validation. Xiaoyue Na: data curation and formal analysis. Jiaxin Liu: investigation and visualization. Junni Wang: investigation and methodology. Cunguo Wang: funding acquisition, supervision, writing-reviewing and editing. Mai-He Li: supervision, writing-reviewing and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Earth and Environment thanks Sarah R. Carrino-Kyke and Ramesha H. Jayaramaiah for their contribution to the peer review of this work. Primary handling editors: Somaparna Ghosh [A peer review file is available].
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhou, Y., Brunner, I., Liu, Z. et al. Mycorrhizal-specific responses of rhizosphere soil properties and fine-root traits to polystyrene microplastic addition in a temperate mixed forest. Commun Earth Environ (2026). https://doi.org/10.1038/s43247-026-03237-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43247-026-03237-0