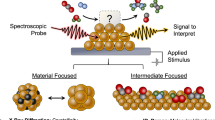
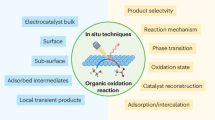
Abstract
Ultraviolet–visible spectroelectrochemistry (SEC) is a powerful and accessible operando technique for investigating redox-active interfaces such as electrodes. Its potential has not been fully realized owing to limitations in sensitivity, acquisition speed and analysis workflows. In this Primer, we describe how recent developments in optics, detection hardware and synchronization methods now allow for high-resolution, data-rich SEC measurements. We focus on practical strategies for building performant SEC set-ups, introduce a formalism based on differential coulometric attenuation for interpreting spectral changes and outline workflows for extracting redox stoichiometries, kinetics and coverage from complex data. Emphasizing process-sensitive over population-sensitive analysis, we show how this approach enables a clearer understanding of dynamic, disordered interfacial systems. Examples are provided from electrocatalysis, particularly the oxygen evolution reaction, but the principles described are broadly applicable. Throughout, we highlight pitfalls, assumptions and design choices to guide researchers looking to implement quantitative SEC in their own work.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$119.00 per year
only $119.00 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
Change history
21 January 2026
A Correction to this paper has been published: https://doi.org/10.1038/s43586-026-00472-9
References
Kim, T., Song, W., Son, D.-Y., Ono, L. K. & Qi, Y. Lithium-ion batteries: outlook on present, future, and hybridized technologies. J. Mater. Chem. A 7, 2942–2964 (2019).
Liu, T. et al. Current challenges and routes forward for nonaqueous lithium-air batteries. Chem. Rev. 120, 6558–6625 (2020).
Burke, M. S., Enman, L. J., Batchellor, A. S., Zou, S. & Boettcher, S. W. Oxygen evolution reaction electrocatalysis on transition metal oxides and (oxy)hydroxides: activity trends and design principles. Chem. Mater. 27, 7549–7558 (2015).
Chung, M. et al. Direct propylene epoxidation via water activation over Pd–Pt electrocatalysts. Science 383, 49–55 (2024).
Chang, W., Jain, A., Rezaie, F. & Manthiram, K. Lithium-mediated nitrogen reduction to ammonia via the catalytic solid–electrolyte interphase. Nat. Catal. 7, 231–241 (2024).
Twight, L. et al. Trace Fe activates perovskite nickelate OER catalysts in alkaline media via redox-active surface Ni species formed during electrocatalysis. J. Catal. 432, 115443 (2024).
Sharpe, L. R., Heineman, W. R. & Elder, R. C. EXAFS spectroelectrochemistry. Chem. Rev. 90, 705–722 (1990).
Zheng, W. Beginner’s guide to Raman spectroelectrochemistry for electrocatalysis study. Chem. Methods 3, e202200042 (2023).
Li, J. et al. Ultrasensitive plasmon‐enhanced infrared spectroelectrochemistry. Angew. Chem. Int. Ed. 63, e202319246 (2024).
Nemes, C. T., Swierk, J. R. & Schmuttenmaer, C. A. A terahertz-transparent electrochemical cell for in situ terahertz spectroelectrochemistry. Anal. Chem. 90, 4389–4396 (2018).
Colburn, A. W., Levey, K. J., O’Hare, D. & Macpherson, J. V. Lifting the lid on the potentiostat: a beginner’s guide to understanding electrochemical circuitry and practical operation. Phys. Chem. Chem. Phys. 23, 8100–8117 (2021).
Selim, S. et al. Impact of oxygen vacancy occupancy on charge carrier dynamics in BiVO4 photoanodes. J. Am. Chem. Soc. 141, 18791–18798 (2019).
Barroso, M. et al. Dynamics of photogenerated holes in surface modified α-Fe2O3 photoanodes for solar water splitting. Proc. Natl Acad. Sci. USA 109, 15640–15645 (2012).
Moss, B. et al. Linking in situ charge accumulation to electronic structure in doped SrTiO3 reveals design principles for hydrogen-evolving photocatalysts. Nat. Mater. 20, 511–517 (2021).
Astuti, Y. et al. Proton-coupled electron transfer of flavodoxin immobilized on nanostructured tin dioxide electrodes: thermodynamics versus kinetics control of protein redox function. J. Am. Chem. Soc. 126, 8001–8009 (2004).
Astuti, Y., Topoglidis, E., Cass, A. G. & Durrant, J. R. Direct spectroelectrochemistry of peroxidases immobilised on mesoporous metal oxide electrodes: towards reagentless hydrogen peroxide sensing. Anal. Chim. Acta 648, 2–6 (2009).
Bevan, A. W. et al. Cyclic voltammetry and spectroelectrochemistry of two common thiophene polymers reveals ion diffusion and polaron wave function extent. Chem. Mater. 37, 1949–1960 (2025).
Guo, J., Moss, B. & Clarke, T. M. Quantifying triplet formation in conjugated polymer/non-fullerene acceptor blends. J. Mater. Chem. A 10, 20874–20885 (2022).
Alsufyani, M. et al. The effect of organic semiconductor electron affinity on preventing parasitic oxidation reactions limiting performance of n‐type organic electrochemical transistors. Adv. Mater. 36, e2403911 (2024).
Moss, B. et al. Cooperative effects drive water oxidation catalysis in cobalt electrocatalysts through the destabilization of intermediates. J. Am. Chem. Soc. 146, 8915–8927 (2024).
Liang, C. et al. Role of electrolyte pH on water oxidation for iridium oxides. J. Am. Chem. Soc. 146, 8928–8938 (2024).
Rao, R. R. et al. Unraveling the role of particle size and nanostructuring on the oxygen evolution activity of Fe-doped NiO. ACS Catal. 14, 11389–11399 (2024).
Liang, C. et al. Unravelling the effects of active site density and energetics on the water oxidation activity of iridium oxides. Nat. Catal. 7, 763–775 (2024).
Lee, S., Moysiadou, A., Chu, Y.-C., Chen, H. M. & Hu, X. Tracking high-valent surface iron species in the oxygen evolution reaction on cobalt iron (oxy)hydroxides. Energy Environ. Sci. https://doi.org/10.1039/d1ee02999a (2021).
Loos, S., Zaharieva, I., Chernev, P., Lißner, A. & Dau, H. Electromodified NiFe alloys as electrocatalysts for water oxidation: mechanistic implications of time‐resolved UV/Vis tracking of oxidation state changes. ChemSusChem 12, 1966–1976 (2019).
Corby, S. et al. Separating bulk and surface processes in NiOx electrocatalysts for water oxidation. Sustain. Energy Fuels 4, 5024–5030 (2020).
Francàs, L. et al. Spectroelectrochemical study of water oxidation on nickel and iron oxyhydroxide electrocatalysts. Nat. Commun. 10, 5208 (2019).
Risch, M. et al. Water oxidation by amorphous cobalt-based oxides: in situ tracking of redox transitions and mode of catalysis. Energy Environ. Sci. 8, 661–674 (2015).
Song, J. et al. A review on fundamentals for designing oxygen evolution electrocatalysts. Chem. Soc. Rev. 49, 2196–2214 (2020).
Dionigi, F. & Strasser, P. NiFe‐based (oxy)hydroxide catalysts for oxygen evolution reaction in non‐acidic electrolytes. Adv. Energy Mater. 6, 1600621 (2016).
Frei, H. Time-resolved vibrational and electronic spectroscopy for understanding how charges drive metal oxide catalysts for water oxidation. J. Phys. Chem. Lett. 13, 7952–7964 (2022).
Hu, B., Kuo, D.-Y., Paik, H., Schlom, D. G. & Suntivich, J. Enthalpy and entropy of oxygen electroadsorption on RuO2(110) in alkaline media. J. Chem. Phys. 152, 094704 (2020).
Kuo, D.-Y. et al. Influence of surface adsorption on the oxygen evolution reaction on IrO2(110). J. Am. Chem. Soc. 139, 3473–3479 (2017).
Laborda, E., García-Martínez, J. & Molina, A. Spectroelectrochemistry for the study of reversible electrode reactions with complex stoichiometries. Electrochem. Commun. 123, 106915 (2021).
Elvers, B. J. et al. Towards operando IR‐ and UV–Vis‐spectro‐electrochemistry: a comprehensive matrix factorisation study on sensitive and transient molybdenum and tungsten mono‐dithiolene complexes. Chem. Methods 1, 22–35 (2021).
Zhai, Y., Zhu, Z., Zhou, S., Zhu, C. & Dong, S. Recent advances in spectroelectrochemistry. Nanoscale 10, 3089–3111 (2018).
Niu, J. & Dong, S. Transmission spectroelectrochemistry. Rev. Anal. Chem. 15, 1–171 (1996).
Goldsmith, Z. K. et al. Characterization of NiFe oxyhydroxide electrocatalysts by integrated electronic structure calculations and spectroelectrochemistry. Proc. Natl Acad. Sci. USA 114, 3050–3055 (2017).
Boyle, W. S. & Smith, G. E. Charge coupled semiconductor devices. Bell Syst. Tech. J. 49, 587–593 (1970).
Fossum, E. R. Active pixel sensors: are CCDs dinosaurs? Charg. Coupled Devices Solid State Opt. Sens. https://doi.org/10.1117/12.148585 (1993).
Hirayama, T. The evolution of CMOS image sensors. In 2013 IEEE Asian Solid-State Circuits Conf. (A-SSCC) 5–8 (IEEE, 2013).
Elgrishi, N. et al. A practical beginner’s guide to cyclic voltammetry. J. Chem. Educ. 95, 197–206 (2018).
Astuti, Y., Topoglidis, E., Gilardi, G. & Durrant, J. R. Cyclic voltammetry and voltabsorptometry studies of redox proteins immobilised on nanocrystalline tin dioxide electrodes. Bioelectrochemistry 63, 55–59 (2004).
Zheng, W. iR compensation for electrocatalysis studies: considerations and recommendations. ACS Energy Lett. 8, 1952–1958 (2023).
Wei, C. et al. Recommended practices and benchmark activity for hydrogen and oxygen electrocatalysis in water splitting and fuel cells. Adv. Mater. 31, 1806296 (2019).
Stevens, M. B., Trang, C. D. M., Enman, L. J., Deng, J. & Boettcher, S. W. Reactive Fe-sites in Ni/Fe (oxy)hydroxide are responsible for exceptional oxygen electrocatalysis activity. J. Am. Chem. Soc. 139, 11361–11364 (2017).
Conway, B. E. & Bourgault, P. L. The electrochemical behavior of the nickel–nickel oxide electrode. Can. J. Chem. 37, 292–307 (1959).
Martinson, C. A., van Schoor, G., Uren, K. R. & Bessarabov, D. Characterisation of a PEM electrolyser using the current interrupt method. Int. J. Hydrog. Energy 39, 20865–20878 (2014).
Watkins, N. B. et al. Resin 3D printing enables accessible electrochemical cell design. Chem. Catal. 4, 101120 (2024).
Benck, J. D., Pinaud, B. A., Gorlin, Y. & Jaramillo, T. F. Substrate selection for fundamental studies of electrocatalysts and photoelectrodes: inert potential windows in acidic, neutral, and basic electrolyte. PLoS ONE 9, e107942 (2014).
Rao, R. R. et al. Spectroelectrochemical analysis of the water oxidation mechanism on doped nickel oxides. J. Am. Chem. Soc. 144, 7622–7633 (2022).
Lerner, J. M. Imaging spectrometer fundamentals for researchers in the biosciences — a tutorial. Cytom. Part A 69A, 712–734 (2006).
Vu, P. et al. Design of prototype scientific CMOS image sensors. High Energy Opt. Infrared Detect. Astron. III https://doi.org/10.1117/12.790229 (2008).
Chen, Z., Dinh, H. N. & Miller, E. Photoelectrochemical water splitting. Standards Exp. Methods Protoc. https://doi.org/10.1007/978-1-4614-8298-7 (2013).
Lewandowski, A., Jakobczyk, P., Galinski, M. & Biegun, M. Self-discharge of electrochemical double layer capacitors. Phys. Chem. Chem. Phys. 15, 8692–8699 (2013).
Nong, H. N. et al. Key role of chemistry versus bias in electrocatalytic oxygen evolution. Nature 587, 408–413 (2020).
Cheng, J. et al. Brønsted−Evans−Polanyi relation of multistep reactions and volcano curve in heterogeneous catalysis. J. Phys. Chem. C 112, 1308–1311 (2008).
Akhade, S. A., Nidzyn, R. M., Rostamikia, G. & Janik, M. J. Using Brønsted−Evans−Polanyi relations to predict electrode potential-dependent activation energies. Catal. Today 312, 82–91 (2018).
Vojvodic, A. et al. On the behavior of Brønsted–Evans–Polanyi relations for transition metal oxides. J. Chem. Phys. 134, 244509 (2011).
Rossmeisl, J., Qu, Z.-W., Zhu, H., Kroes, G.-J. & Nørskov, J. K. Electrolysis of water on oxide surfaces. J. Electroanal. Chem. 607, 83–89 (2007).
Man, I. C. et al. Universality in oxygen evolution electrocatalysis on oxide surfaces. ChemCatChem 3, 1159–1165 (2011).
Frydendal, R., Paoli, E. A., Chorkendorff, I., Rossmeisl, J. & Stephens, I. E. L. Toward an active and stable catalyst for oxygen evolution in acidic media: Ti‐stabilized MnO2. Adv. Energy Mater. 5, 1500991 (2015).
Seh, Z. W. et al. Combining theory and experiment in electrocatalysis: insights into materials design. Science 355, eaad4998 (2017).
Scott, S. B. et al. The low overpotential regime of acidic water oxidation part I: the importance of O2 detection. Energy Environ. Sci. 15, 1977–1987 (2022).
Burke, M. S., Kast, M. G., Trotochaud, L., Smith, A. M. & Boettcher, S. W. Cobalt–iron (oxy)hydroxide oxygen evolution electrocatalysts: the role of structure and composition on activity, stability, and mechanism. J. Am. Chem. Soc. 137, 3638–3648 (2015).
Aggarwal, S. et al. PyTASER: simulating transient absorption spectroscopy (TAS) for crystals from first principles. J. Open Source Softw. 9, 5999 (2024).
Guevarra, D. et al. Orchestrating nimble experiments across interconnected labs. Digit. Discov. 2, 1806–1812 (2023).
Acknowledgements
B.M. acknowledges the Lindemann Trust and Schmidt Sciences for funding. He is also indebted to K. K. Lim and X. Li for thoughtful discussion that clarified many points on this work. J.R.D. and I.E.L.S. acknowledge the BP International Centre for Advanced Materials (bp-ICAM) as well as the EPSRC grant EP/W033232/1, which made this research possible. This project was supported by the Royal Academy of Engineering under the Research Fellowship programme (R.R.R.). C.L. acknowledges the Imperial College London and China Scholarship Council for the IC–CSC joint scholarship.
Author information
Authors and Affiliations
Contributions
Introduction: B.M., C.L., L.G.V., S.S., R.R.R., I.E.L.S. and J.R.D.; Experimentation: B.M., C.L., A.C., L.G.V., S.S., K.M., R.R.R., I.E.L.S. and J.R.D.; Results: B.M., C.L., A.C., S.S., K.M., A.W., R.R.R., I.E.L.S., J.R.D. and R.J.R.J.; Applications: B.M., C.L., A.C., S.S., K.M., R.R.R., I.E.L.S. and J.R.D.; Reproducibility and data deposition: B.M., C.L., S.S., R.R.R., I.E.L.S. and J.R.D.; Limitations and optimizations: B.M., C.L., A.C., L.G.V., S.S., K.M., A.W., R.R.R., I.E.L.S. and J.R.D.; Outlook: B.M., C.L., A.C., L.G.V., S.S., R.R.R., I.E.L.S. and J.R.D.
Corresponding author
Ethics declarations
Competing interests
A.C. is an employee of Oxford Instruments Andor (OIA) and contributed his perspective to discussions of the detector technologies with respect to the SEC spectroscopic methodology. No financial or commercial support has been provided by OIA. All other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Methods Primers thanks Evgenia Dmitrieva, Marcel Risch and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
GitHub archive: https://github.com/BenjaminSMoss/NRMP
ixdat: https://ixdat.readthedocs.io
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Moss, B., Liang, C., Carpenter, A. et al. Operando ultraviolet–visible optical spectroelectrochemistry of surfaces. Nat Rev Methods Primers 5, 73 (2025). https://doi.org/10.1038/s43586-025-00445-4
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s43586-025-00445-4