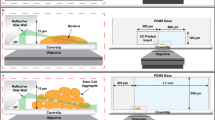
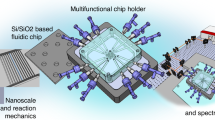
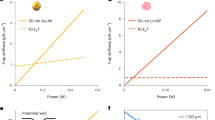
Abstract
Fluidic force microscopy is a versatile bionanotechnology platform that integrates atomic force microscopy with microfluidic probes. This hybrid approach enables precise measurement and application of forces across sub-nanonewton to micronewton ranges while simultaneously dispensing or sampling sub-femtolitre to picolitre volumes, all with real-time optical visualization at sub-micrometre resolution. In recent years, fluidic force microscopy has emerged as an enabling tool for minimally invasive single-cell manipulation, subcellular analysis and high-resolution nanoprinting applications. This Primer describes the fundamental principles underlying the combination of atomic force microscopy with microchannelled cantilevers, providing a comprehensive framework for understanding the unique capabilities of fluidic force microscopy and its rapidly expanding range of applications in biological research and nanotechnology.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$119.00 per year
only $119.00 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Meister, A. et al. FluidFM: combining atomic force microscopy and nanofluidics in a universal liquid delivery system for single cell applications and beyond. Nano Lett. 9, 2501–2507 (2009).
Barber, M. A. A new method of isolating micro-organisms. J. Kans. Med. Soc. 4, 489–494 (1904).
Korzh, V. & Strähle, U. Marshall Barber and the century of microinjection: from cloning of bacteria to cloning of everything. Differentiation 70, 221–226 (2002).
Thomas, K. R. & Capecchi, M. R. Introduction of homologous DNA sequences into mammalian cells induces mutations in the cognate gene. Nature 324, 34–38 (1986).
Eberwine, J., Sul, J.-Y., Bartfai, T. & Kim, J. The promise of single-cell sequencing. Nat. Methods 11, 25–27 (2014).
Marcuccio, F. et al. Single-cell nanobiopsy enables multigenerational longitudinal transcriptomics of cancer cells. Sci. Adv. 10, eadl0515 (2024).
Neher, E. & Sakmann, B. Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature 260, 799–802 (1976).
Momotenko, D., Page, A., Adobes-Vidal, M. & Unwin, P. R. Write–read 3D patterning with a dual-channel nanopipette. ACS Nano 10, 8871–8878 (2016).
Hengsteler, J. et al. Bringing electrochemical three-dimensional printing to the nanoscale. Nano Lett. 21, 9093–9101 (2021).
Xu, X. et al. The new era of high-throughput nanoelectrochemistry. Anal. Chem. 95, 319–356 (2023).
Hansma, P. K., Drake, B., Marti, O., Gould, S. A. C. & Prater, C. B. The scanning ion-conductance microscope. Science 243, 641–643 (1989).
Korchev, Y. E., Bashford, C. L., Milovanovic, M., Vodyanoy, I. & Lab, M. J. Scanning ion conductance microscopy of living cells. Biophys. J. 73, 653–658 (1997).
Novak, P. et al. Nanoscale live-cell imaging using hopping probe ion conductance microscopy. Nat. Methods 6, 279–281 (2009).
Long, B., Li, L., Knoblich, U., Zeng, H. & Peng, H. 3D image-guided automatic pipette positioning for single cell experiments in vivo. Sci. Rep. 5, 18426 (2015).
Proksch, R., Lal, R., Hansma, P. K., Morse, D. & Stucky, G. Imaging the internal and external pore structure of membranes in fluid: tappingmode scanning ion conductance microscopy. Biophys. J. 71, 2155–2157 (1996).
Lewis, A. et al. Fountain pen nanochemistry: atomic force control of chrome etching. Appl. Phys. Lett. 75, 2689–2691 (1999).
Drake, B., Randall, C., Bridges, D. & Hansma, P. K. A new ion sensing deep atomic force microscope. Rev. Sci. Instrum. 85, 083706 (2014).
Lu, Z., Chen, P. C. Y., Nam, J., Ge, R. & Lin, W. A micromanipulation system with dynamic force-feedback for automatic batch microinjection. J. Micromech. Microeng. 17, 314 (2007).
Ito, S. & Iwata, F. Nanometer-scale deposition of metal plating using a nanopipette probe in liquid condition. Jpn. J. Appl. Phys. 50, 08LB15 (2011).
An, S. et al. Nanopipette combined with quartz tuning fork-atomic force microscope for force spectroscopy/microscopy and liquid delivery-based nanofabrication. Rev. Sci. Instrum. 85, 033702 (2014).
Ma, Y. et al. Label-free robotic mitochondrial biopsy. Sci. Adv. 11, eadx4289 (2025).
Binnig, G., Quate, C. F. & Gerber, C. H. Atomic force microscope. Phys. Rev. Lett. 56, 930–933 (1986).
Bian, K. et al. Scanning probe microscopy. Nat. Rev. Methods Primers 1, 36 (2021).
Voigtländer, B. Atomic Force Microscopy 2nd edn (Springer, 2019).
Wendel, M. et al. Nanolithography with an atomic force microscope. Superlattices Microstruct. 20, 349–356 (1996).
Jaschke, M. & Butt, H.-J. Deposition of organic material by the tip of a scanning force microscope. Langmuir 11, 1061–1064 (1995).
Piner, R. D., Zhu, J., Xu, F., Hong, S. & Mirkin, C. A. ‘Dip-pen’ nanolithography. Science 283, 661–663 (1999).
Meister, A. et al. Nanoscale dispensing of liquids through cantilevered probes. Microelectron. Eng. 67–68, 644–650 (2003).
Meister, A., Liley, M., Brugger, J., Pugin, R. & Heinzelmann, H. Nanodispenser for attoliter volume deposition using atomic force microscopy probes modified by focused-ion-beam milling. Appl. Phys. Lett. 85, 6260–6262 (2004).
Deladi, S. et al. Micromachined fountain pen for atomic force microscope-based nanopatterning. Appl. Phys. Lett. 85, 5361–5363 (2004).
Moldovan, N., Kim, K.-H. & Espinosa, H. D. Design and fabrication of a novel microfluidic nanoprobe. J. Microelectromechanical Syst. 15, 204–213 (2006).
Hug, T. S., Biss, T., de Rooij, N. F. & Staufer, U. Generic fabrication technology for transparent and suspended microfluidic and nanofluidic channels. In Proc. 13th International Conference on Solid-State Sensors, Actuators and Microsystems, 2005. Digest of Technical Papers. TRANSDUCERS ‘05, Vol. 2 1191–1194 (IEEE, 2005).
Kato, N., Kawashima, T., Shibata, T., Mineta, T. & Makino, E. Micromachining of a newly designed AFM probe integrated with hollow microneedle for cellular function analysis. Microelectron. Eng. 87, 1185–1189 (2010).
Delamarche, E. & Kaigala, G. V. (eds) Open-Space Microfluidics: Concepts, Implementations, Applications 1st edn (Wiley-VCH, 2018).
Ossola, D. et al. Force-controlled patch clamp of beating cardiac cells. Nano Lett. 15, 1743–1750 (2015).
Hirt, L. et al. Local surface modification via confined electrochemical deposition with FluidFM. RSC Adv. 5, 84517–84522 (2015).
Saftics, A. et al. Biomimetic dextran-based hydrogel layers for cell micropatterning over large areas using the FluidFM BOT technology. Langmuir 35, 2412–2421 (2019).
Sztilkovics, M. et al. Single-cell adhesion force kinetics of cell populations from combined label-free optical biosensor and robotic fluidic force microscopy. Sci. Rep. 10, 61 (2020).
Nagy, Á. G. et al. Population distributions of single-cell adhesion parameters during the cell cycle from high-throughput robotic fluidic force microscopy. Sci. Rep. 12, 7747 (2022).
Nagy, Á. G., Székács, I., Bonyár, A. & Horvath, R. Cell-substratum and cell-cell adhesion forces and single-cell mechanical properties in mono- and multilayer assemblies from robotic fluidic force microscopy. Eur. J. Cell Biol. 101, 151273 (2022).
Kovács, K. D. et al. Nanoinjection of extracellular vesicles to single live cells by robotic fluidic force microscopy. J. Extracell. Vesicles 12, e12388 (2023).
Gerecsei, T. et al. Adhesion force measurements on functionalized microbeads: An in-depth comparison of computer controlled micropipette and fluidic force microscopy. J. Colloid Interface Sci. 555, 245–253 (2019).
Ungai-Salánki, R. et al. Single-cell adhesion strength and contact density drops in the M phase of cancer cells. Sci. Rep. 11, 18500 (2021).
Lelek, M. et al. Single-molecule localization microscopy. Nat. Rev. Methods Primers 1, 39 (2021).
Meyer, G. & Amer, N. M. Novel optical approach to atomic force microscopy. Appl. Phys. Lett. 53, 1045–1047 (1988).
Roder, P. & Hille, C. A multifunctional frontloading approach for repeated recycling of a pressure-controlled AFM micropipette. PLoS ONE 10, e0144157 (2015).
Zare-Eelanjegh, E., Lewis, R. T., Lüchtefeld, I., Kutay, U. & Zambelli, T. Quantifying intracellular mechanosensitive response upon spatially defined mechano-chemical triggering. eLife https://doi.org/10.7554/eLife.107220.1 (2025).
Kenausis, G. L. et al. Poly(l-lysine)-g-poly(ethylene glycol) layers on metal oxide surfaces: attachment mechanism and effects of polymer architecture on resistance to protein adsorption. J. Phys. Chem. B 104, 3298–3309 (2000).
Huang, N.-P. et al. Poly(l-lysine)-g-poly(ethylene glycol) layers on metal oxide surfaces: surface-analytical characterization and resistance to serum and fibrinogen adsorption. Langmuir 17, 489–498 (2001).
Weydert, S. et al. Easy to apply polyoxazoline-based coating for precise and long-term control of neural patterns. Langmuir 33, 8594–8605 (2017).
Guillaume-Gentil, O. et al. Force-controlled fluidic injection into single cell nuclei. Small 9, 1904–1907 (2013).
Guillaume-Gentil, O. et al. Injection into and extraction from single fungal cells. Commun. Biol. 5, 180 (2022).
Potthoff, E. et al. Rapid and serial quantification of adhesion forces of yeast and mammalian cells. PLoS ONE 7, e52712 (2012).
Guillaume-Gentil, O., Zambelli, T. & Vorholt, J. A. Isolation of single mammalian cells from adherent cultures by fluidic force microscopy. Lab Chip 14, 402–414 (2014).
Dehullu, J. et al. Fluidic force microscopy demonstrates that homophilic adhesion by Candida albicans Als proteins is mediated by amyloid bonds between cells. Nano Lett. 19, 3846–3853 (2019).
Potthoff, E., Ossola, D., Zambelli, T. & Vorholt, J. A. Bacterial adhesion force quantification by fluidic force microscopy. Nanoscale 7, 4070–4079 (2015).
Guo, Y. et al. Matrix stiffness modulates tip cell formation through the p-PXN-Rac1-YAP signaling axis. Bioact. Mater. 7, 364–376 (2022).
Chala, N. et al. 4D force detection of cell adhesion and contractility. Nano Lett. 23, 2467–2475 (2023).
Ernst, C. et al. Direct Salmonella injection into enteroid cells allows the study of host–pathogen interactions in the cytosol with high spatiotemporal resolution. PLoS Biol. 22, e3002597 (2024).
Antony, J. S. et al. Accelerated generation of gene-engineered monoclonal CHO cell lines using FluidFM nanoinjection and CRISPR/Cas9. Biotechnol. J. 19, e2300505 (2024).
Giger, G. H. et al. Inducing novel endosymbioses by implanting bacteria in fungi. Nature 635, 415–422 (2024).
Pan, F. et al. Uncoupling bacterial attachment on and detachment from polydimethylsiloxane surfaces through empirical and simulation studies. J. Colloid Interface Sci. 622, 419–430 (2022).
Potthoff, E. et al. Toward a rational design of surface textures promoting endothelialization. Nano Lett. 14, 1069–1079 (2014).
Mathelié-Guinlet, M. et al. Single-cell fluidic force microscopy reveals stress-dependent molecular interactions in yeast mating. Commun. Biol. 4, 33 (2021).
D’Costa, N. P. & Hoh, J. H. Calibration of optical lever sensitivity for atomic force microscopy. Rev. Sci. Instrum. 66, 5096–5097 (1995).
Nagy, Á. G., Kámán, J., Horváth, R. & Bonyár, A. Spring constant and sensitivity calibration of FluidFM micropipette cantilevers for force spectroscopy measurements. Sci. Rep. 9, 10287 (2019).
Viljoen, A. et al. Force spectroscopy of single cells using atomic force microscopy. Nat. Rev. Methods Primers 1, 63 (2021).
Sader, J. E. Frequency response of cantilever beams immersed in viscous fluids with applications to the atomic force microscope. J. Appl. Phys. 84, 64–76 (1998).
Sader, J. E. et al. Spring constant calibration of atomic force microscope cantilevers of arbitrary shape. Rev. Sci. Instrum. 83, 103705 (2012).
Payam, A. F., Trewby, W. & Voïtchovsky, K. Determining the spring constant of arbitrarily shaped cantilevers in viscous environments. Appl. Phys. Lett. 112, 083101 (2018).
Bonyár, A., Nagy, Á. G., Gunstheimer, H., Fläschner, G. & Horvath, R. Hydrodynamic function and spring constant calibration of FluidFM micropipette cantilevers. Microsyst. Nanoeng. 10, 26 (2024).
Helfricht, N., Mark, A., Dorwling-Carter, L., Zambelli, T. & Papastavrou, G. Extending the limits of direct force measurements: colloidal probes from sub-micron particles. Nanoscale 9, 9491–9501 (2017).
Sittl, S., Helfricht, N. & Papastavrou, G. Contactless calibration of microchanneled AFM cantilevers for fluidic force microscopy. View 5, 20230063 (2024).
Mark, A., Helfricht, N., Rauh, A., Karg, M. & Papastavrou, G. The next generation of colloidal probes: a universal approach for soft and ultra-small particles. Small 15, 1902976 (2019).
Mittelviefhaus, M., Müller, D. B., Zambelli, T. & Vorholt, J. A. A modular atomic force microscopy approach reveals a large range of hydrophobic adhesion forces among bacterial members of the leaf microbiota. ISME J. 13, 1878–1882 (2019).
Hofherr, L., Müller-Renno, C. & Ziegler, C. FluidFM as a tool to study adhesion forces of bacteria - optimization of parameters and comparison to conventional bacterial probe scanning force spectroscopy. PLoS ONE 15, e0227395 (2020).
Manoharan, G. et al. FluidFM deposition of semicondutor quantum dots from aqueous dispersions. Nano Select 6, e70011 (2025).
Dörig, P. et al. Exchangeable colloidal AFM probes for the quantification of irreversible and long-term interactions. Biophys. J. 105, 463–472 (2013).
Specht, A., Krämer, D., Helfricht, N. & Papastavrou, G. How much data are enough? Toward statistically robust adhesion experiments by atomic force microscopy. Langmuir 41, 6515–6527 (2025).
Guillaume-Gentil, O. et al. Tunable single-cell extraction for molecular analyses. Cell 166, 506–516 (2016).
Chen, W. et al. Live-seq enables temporal transcriptomic recording of single cells. Nature 608, 733–740 (2022).
Gäbelein, C. G. et al. Mitochondria transplantation between living cells. PLoS Biol. 20, e3001576 (2022).
Mark, A. et al. Electrokinetics in micro-channeled cantilevers: extending the toolbox for reversible colloidal probes and AFM-based nanofluidics. Sci. Rep. 9, 20294 (2019).
Aramesh, M. et al. Localized detection of ions and biomolecules with a force-controlled scanning nanopore microscope. Nat. Nanotechnol. 14, 791–798 (2019).
Rosenbluth, M. J., Lam, W. A. & Fletcher, D. A. Force microscopy of nonadherent cells: a comparison of leukemia cell deformability. Biophys. J. 90, 2994–3003 (2006).
Feng, Y. & Li, M. Micropipette-assisted atomic force microscopy for single-cell 3D manipulations and nanomechanical measurements. Nanoscale 15, 13346–13358 (2023).
Lüchtefeld, I. et al. Dissecting cell membrane tension dynamics and its effect on Piezo1-mediated cellular mechanosensitivity using force-controlled nanopipettes. Nat. Methods 21, 1063–1073 (2024).
Hertz, H. Über die Berührung fester elastischer Körper. J. Reine Angew. Math. 92, 156–171 (1882).
Guz, N., Dokukin, M., Kalaparthi, V. & Sokolov, I. If cell mechanics can be described by elastic modulus: study of different models and probes used in indentation experiments. Biophys. J. 107, 564–575 (2014).
Krieg, M. et al. Atomic force microscopy-based mechanobiology. Nat. Rev. Phys. 1, 41–57 (2019).
Kontomaris, S. V., Georgakopoulos, A., Malamou, A. & Stylianou, A. The average Young’s modulus as a physical quantity for describing the depth-dependent mechanical properties of cells. Mech. Mater. 158, 103846 (2021).
Kontomaris, S. V., Malamou, A. & Stylianou, A. The hertzian theory in AFM nanoindentation experiments regarding biological samples: overcoming limitations in data processing. Micron 155, 103228 (2022).
Mendová, K., Otáhal, M., Drab, M. & Daniel, M. Size matters: rethinking Hertz model interpretation for cell mechanics using AFM. Int. J. Mol. Sci. 25, 7186 (2024).
Garcia, R. Nanomechanical mapping of soft materials with the atomic force microscope: methods, theory and applications. Chem. Soc. Rev. 49, 5850–5884 (2020).
Lin, D. C., Dimitriadis, E. K. & Horkay, F. Robust strategies for automated AFM force curve analysis—i. non-adhesive indentation of soft, inhomogeneous materials. J. Biomech. Eng. 129, 430–440 (2007).
Crick, S. L. & Yin, F. C.-P. Assessing micromechanical properties of cells with atomic force microscopy: importance of the contact point. Biomech. Model. Mechanobiol. 6, 199–210 (2007).
Gavara, N. Combined strategies for optimal detection of the contact point in AFM force-indentation curves obtained on thin samples and adherent cells. Sci. Rep. 6, 21267 (2016).
Lüchtefeld, I. et al. Elasticity spectra as a tool to investigate actin cortex mechanics. J. Nanobiotechnology 18, 147 (2020).
Helenius, J., Heisenberg, C.-P., Gaub, H. E. & Muller, D. J. Single-cell force spectroscopy. J. Cell Sci. 121, 1785–1791 (2008).
Franz, C. M. & Puech, P.-H. Atomic force microscopy: a versatile tool for studying cell morphology, adhesion and mechanics. Cell. Mol. Bioeng. 1, 289–300 (2008).
Moreno-Cencerrado, A. et al. Investigating cell-substrate and cell–cell interactions by means of single-cell-probe force spectroscopy. Microsc. Res. Tech. 80, 124–130 (2017).
Grüter, R. R., Vörös, J. & Zambelli, T. FluidFM as a lithography tool in liquid: spatially controlled deposition of fluorescent nanoparticles. Nanoscale 5, 1097–1104 (2013).
Zhang, X. et al. Multimodal mapping of electrical and mechanical latency of human-induced pluripotent stem cell-derived cardiomyocyte layers. ACS Nano 18, 24060–24075 (2024).
Andreassen, P. R. et al. NAIP/NLRC4 inflammasome dynamics in murine enteroids are tuned by NAIP ligand concentration and epithelial cell differentiation. Cell Rep. 44, 116143 (2025).
Gäbelein, C. G., Reiter, M. A., Ernst, C., Giger, G. H. & Vorholt, J. A. Engineering endosymbiotic growth of E. coli in mammalian cells. ACS Synth. Biol. 11, 3388–3396 (2022).
Guillaume-Gentil, O. et al. Single-cell mass spectrometry of metabolites extracted from live cells by fluidic force microscopy. Anal. Chem. 89, 5017–5023 (2017).
Xu, S., Miller, S., Laibinis, P. E. & Liu, G. Fabrication of nanometer scale patterns within self-assembled monolayers by nanografting. Langmuir 15, 7244–7251 (1999).
Xu, S., Amro, N. A. & Liu, G.-Y. Characterization of AFM tips using nanografting. Appl. Surf. Sci. 175–176, 649–655 (2001).
Liu, J.-F., Cruchon-Dupeyrat, S., Garno, J. C., Frommer, J. & Liu, G.-Y. Three-dimensional nanostructure construction via nanografting: positive and negative pattern transfer. Nano Lett. 2, 937–940 (2002).
Hong, S., Zhu, J. & Mirkin, C. A. Multiple ink nanolithography: toward a multiple-pen nano-plotter. Science 286, 523–525 (1999).
Fang, A., Dujardin, E. & Ondarçuhu, T. Control of droplet size in liquid nanodispensing. Nano Lett. 6, 2368–2374 (2006).
Fabié, L. & Ondarçuhu, T. Writing with liquid using a nanodispenser: spreading dynamics at the sub-micron scale. Soft Matter 8, 4995–5001 (2012).
Fabié, L. et al. Direct patterning of nanoparticles and biomolecules by liquid nanodispensing. Nanoscale 7, 4497–4504 (2015).
Dermutz, H. et al. Local polymer replacement for neuron patterning and in situ neurite guidance. Langmuir 30, 7037–7046 (2014).
Grüter, R. R., Dielacher, B., Hirt, L., Vörös, J. & Zambelli, T. Patterning gold nanoparticles in liquid environment with high ionic strength for local fabrication of up to 100 μm long metallic interconnections. Nanotechnology 26, 175301 (2015).
Zhang, J. et al. Controlled molecular assembly via dynamic confinement of solvent. J. Phys. Chem. Lett. 9, 6232–6237 (2018).
Zhang, J. et al. New means to control molecular assembly. J. Phys. Chem. C 124, 6405–6412 (2020).
Berganza, E. & Hirtz, M. Direct-write patterning of biomimetic lipid membranes in situ with FluidFM. ACS Appl. Mater. Interfaces 13, 50774–50784 (2021).
Apte, G., Hirtz, M. & Nguyen, T.-H. FluidFM-based fabrication of nanopatterns: promising surfaces for platelet storage application. ACS Appl. Mater. Interfaces 14, 24133–24143 (2022).
Helfricht, N. et al. Writing with fluid: structuring hydrogels with micrometer precision by AFM in combination with nanofluidics. Small 13, 1700962 (2017).
Hirt, L. et al. Template-free 3D microprinting of metals using a force-controlled nanopipette for layer-by-layer electrodeposition. Adv. Mater. 28, 2311–2315 (2016).
Ercolano, G. et al. Additive manufacturing of sub-micron to sub-mm metal structures with hollow AFM cantilevers. Micromachines 11, 6 (2020).
Ercolano, G. et al. Multiscale additive manufacturing of metal microstructures. Adv. Eng. Mater. 22, 1900961 (2020).
van Nisselroy, C., Shen, C., Zambelli, T. & Momotenko, D. Electrochemical 3D printing of silver and nickel microstructures with FluidFM. Addit. Manuf. 53, 102718 (2022).
Shen, C. et al. Electrochemical 3D printing of Ni–Mn and Ni–Co alloy with FluidFM. Nanotechnology 33, 265301 (2022).
Pratama, K. et al. From 2D to 3D electrochemical microfabrication of nickel architectures at room temperature: Synthesis and characterization of microstructure and mechanical properties. Addit. Manuf. 88, 104251 (2024).
Ventrici de Souza, J. et al. Three-dimensional nanoprinting via direct delivery. J. Phys. Chem. B 122, 956–962 (2018).
Huang, Y. et al. Controlled assembly of lipid molecules via regulating transient spatial confinement. Chemistry 6, 1287–1300 (2024).
Stiefel, P. et al. Cooperative vaccinia infection demonstrated at the single-cell level using FluidFM. Nano Lett. 12, 4219–4227 (2012).
Aebersold, M. J. et al. Local chemical stimulation of neurons with the fluidic force microscope (FluidFM). ChemPhysChem 19, 1234–1244 (2018).
Mulder, E. J., Moser, B., Delgado, J., Steinhardt, R. C. & Esser-Kahn, A. P. Evidence of collective influence in innate sensing using fluidic force microscopy. Front. Immunol. 15, 1340384 (2024).
Senden, T. J. Force microscopy and surface interactions. Curr. Opin. Colloid Interface Sci. 6, 95–101 (2001).
Butt, H.-J., Cappella, B. & Kappl, M. Force measurements with the atomic force microscope: technique, interpretation and applications. Surf. Sci. Rep. 59, 1–152 (2005).
Butt, H.-J. et al. Steric forces measured with the atomic force microscope at various temperatures. Langmuir 15, 2559–2565 (1999).
VanLandingham, M. R., Villarrubia, J. S., Guthrie, W. F. & Meyers, G. F. Nanoindentation of polymers: an overview. Macromol. Symp. 167, 15–44 (2001).
Ducker, W. A., Senden, T. J. & Pashley, R. M. Direct measurement of colloidal forces using an atomic force microscope. Nature 353, 239–241 (1991).
Butt, H.-J. Measuring electrostatic, van der Waals, and hydration forces in electrolyte solutions with an atomic force microscope. Biophys. J. 60, 1438–1444 (1991).
Specht, A. et al. High-throughput mechanical characterization of single microgel particles by fluidic force microscopy. Small 21, e05367 (2025).
Rosales, A. B., Causserand, C., Coetsier, C. & Formosa-Dague, C. Probing the reduction of adhesion forces between biofilms and anti-biofouling filtration membrane surfaces using FluidFM technology. Colloids Surf. B Biointerfaces 234, 113701 (2024).
Helfricht, N., Doblhofer, E., Duval, J. F. L., Scheibel, T. & Papastavrou, G. Colloidal properties of recombinant spider silk protein particles. J. Phys. Chem. C 120, 18015–18027 (2016).
Schwan, L. & Bröckel, U. First approach using fluidic force microscopy (FluidFM®) to measure adhesion forces between droplets and flat/rough surfaces immersed in water. Processes 12, 99 (2024).
Demir, I. et al. Probing the interactions between air bubbles and (bio)interfaces at the nanoscale using FluidFM technology. J. Colloid Interface Sci. 604, 785–797 (2021).
Emiroglu, D. B. et al. Building block properties govern granular hydrogel mechanics through contact deformations. Sci. Adv. 8, eadd8570 (2022).
McGrath, J. S. et al. Deformability assessment of waterborne protozoa using a microfluidic-enabled force microscopy probe. PLoS ONE 11, e0150438 (2016).
Ossola, D., Dörig, P., Vörös, J., Zambelli, T. & Vassalli, M. Serial weighting of micro-objects with resonant microchanneled cantilevers. Nanotechnology 27, 415502 (2016).
Dörig, P. et al. Force-controlled spatial manipulation of viable mammalian cells and micro-organisms by means of FluidFM technology. Appl. Phys. Lett. 97, 023701 (2010).
Sancho, A., Vandersmissen, I., Craps, S., Luttun, A. & Groll, J. A new strategy to measure intercellular adhesion forces in mature cell-cell contacts. Sci. Rep. 7, 46152 (2017).
Sancho, A. et al. Cell adhesion assessment reveals a higher force per contact area on fibrous structures compared to flat substrates. ACS Biomater. Sci. Eng. 8, 649–658 (2022).
Weigl, F., Blum, C., Sancho, A. & Groll, J. Correlative analysis of intra- versus extracellular cell detachment events via the alignment of optical imaging and detachment force quantification. Adv. Mater. Technol. 7, 2200195 (2022).
Chala, N. et al. Mechanical fingerprint of senescence in endothelial cells. Nano Lett. 21, 4911–4920 (2021).
Buisson, J. et al. Reverse mechanotransduction: driving chromatin compaction to decompaction increases cell adhesion strength and contractility. Nano Lett. 24, 4279–4290 (2024).
Cui, Y. et al. Global miRNA dosage control of embryonic germ layer specification. Nature 593, 602–606 (2021).
Gassler, T. et al. Induced endosymbiosis between a fungus and bacterium reveals a shift from antagonism to commensalism. Nat. Commun. 16, 10717 (2025).
Jentsch, T. J., Hübner, C. A. & Fuhrmann, J. C. Ion channels: function unravelled by dysfunction. Nat. Cell Biol. 6, 1039–1047 (2004).
Coste, B. et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 330, 55–60 (2010).
Smith, S. L., Smith, I. T., Branco, T. & Häusser, M. Dendritic spikes enhance stimulus selectivity in cortical neurons in vivo. Nature 503, 115–120 (2013).
Zhu, C., Huang, K., Siepser, N. P. & Baker, L. A. Scanning ion conductance microscopy. Chem. Rev. 121, 11726–11768 (2021).
Ossola, D. et al. Simultaneous scanning ion conductance microscopy and atomic force microscopy with microchanneled cantilevers. Phys. Rev. Lett. 115, 238103 (2015).
Dorwling-Carter, L. et al. Simultaneous scanning ion conductance and atomic force microscopy with a nanopore: effect of the aperture edge on the ion current images. J. Appl. Phys. 124, 174902 (2018).
Schlotter, T. et al. Force-controlled formation of dynamic nanopores for single-biomolecule sensing and single-cell secretomics. ACS Nano 14, 12993–13003 (2020).
Schlotter, T. et al. Aptamer-functionalized interface nanopores enable amino acid-specific peptide detection. ACS Nano 18, 6286–6297 (2024).
Uhlén, M. et al. The human secretome. Sci. Signal. 12, eaaz0274 (2019).
te Riet, J. et al. Interlaboratory round robin on cantilever calibration for AFM force spectroscopy. Ultramicroscopy 111, 1659–1669 (2011).
Hosaka, S., Etoh, K., Kikukawa, A. & Koyanagi, H. Megahertz silicon atomic force microscopy (AFM) cantilever and high-speed readout in AFM-based recording. J. Vac. Sci. Technol. B 18, 94–99 (2000).
Dzedzickis, A., Rožėnė, J., Bučinskas, V., Viržonis, D. & Morkvėnaitė-Vilkončienė, I. Characteristics and functionality of cantilevers and scanners in atomic force microscopy. Materials 16, 6379 (2023).
Korayem, M. H., Saraie, M. B. & Saraee, M. B. Analysis the effect of different geometries of AFM’s cantilever on the dynamic behavior and the critical forces of three-dimensional manipulation. Ultramicroscopy 175, 9–24 (2017).
Glaubitz, M. et al. A novel contact model for AFM indentation experiments on soft spherical cell-like particles. Soft Matter 10, 6732–6741 (2014).
Raßmann, N. et al. Determining the elastic modulus of microgel particles by nanoindentation. ACS Appl. Nano Mater. 8, 5383–5398 (2025).
Bay, J., Bouwstra, S., Laegsgaard, E. & Hansen, O. Micromachined AFM transducer with differential capacitive read-out. J. Micromech. Microeng. 5, 161 (1995).
Brugger, J., Buser, R. A. & de Rooij, N. F. Micromachined atomic force microprobe with integrated capacitive read-out. J. Micromech. Microeng. 2, 218 (1992).
Itoh, T. & Suga, T. Development of a force sensor for atomic force microscopy using piezoelectric thin films. Nanotechnology 4, 218 (1993).
Shibata, T., Unno, K., Makino, E., Ito, Y. & Shimada, S. Characterization of sputtered ZnO thin film as sensor and actuator for diamond AFM probe. Sens. Actuators A Phys. 102, 106–113 (2002).
Shin, C., Jeon, I., Khim, Z. G., Hong, J. W. & Nam, H. Study of sensitivity and noise in the piezoelectric self-sensing and self-actuating cantilever with an integrated Wheatstone bridge circuit. Rev. Sci. Instrum. 81, 035109 (2010).
Tortonese, M., Barrett, R. C. & Quate, C. F. Atomic resolution with an atomic force microscope using piezoresistive detection. Appl. Phys. Lett. 62, 834–836 (1993).
Linnemann, R., Gotszalk, T., Hadjiiski, L. & Rangelow, I. W. Characterization of a cantilever with an integrated deflection sensor. Thin Solid Films 264, 159–164 (1995).
Dukic, M., Adams, J. D. & Fantner, G. E. Piezoresistive AFM cantilevers surpassing standard optical beam deflection in low noise topography imaging. Sci. Rep. 5, 16393 (2015).
Han, H. et al. Integration of silver nanowires into SU-8 hollow cantilevers for piezoresistive-based sensing. Sens. Actuators A: Phys. 301, 111748 (2020).
Hosseini, N. et al. A polymer–semiconductor–ceramic cantilever for high-sensitivity fluid-compatible microelectromechanical systems. Nat. Electron. 7, 567–575 (2024).
Suchyna, T. M., Markin, V. S. & Sachs, F. Biophysics and structure of the patch and the gigaseal. Biophys J. 97, 738–747 (2009).
Priel, A., Gil, Z., Moy, V. T., Magleby, K. L. & Silberberg, S. D. Ionic requirements for membrane-glass adhesion and giga seal formation in patch-clamp recording. Biophys. J. 92, 3893–3900 (2007).
Böhle, T. & Benndorf, K. Facilitated giga-seal formation with a just originated glass surface. Pflügers Arch. 427, 487–491 (1994).
Malboubi, M., Gu, Y. & Jiang, K. Experimental and simulation study of the effect of pipette roughness on giga-seal formation in patch clamping. Microelectron. Eng. 87, 778–781 (2010).
Novak, P. et al. Imaging single nanoparticle interactions with human lung cells using fast ion conductance microscopy. Nano Lett. 14, 1202–1207 (2014).
Vélez-Ortega, A. C. et al. High-speed hopping probe scanning ion conductance microscopy. Biophys. J. 106, 797a–798a (2014).
Watanabe, S., Kitazawa, S., Sun, L., Kodera, N. & Ando, T. Development of high-speed ion conductance microscopy. Rev. Sci. Instrum. 90, 123704 (2019).
Leitao, S. M. et al. Time-resolved scanning ion conductance microscopy for three-dimensional tracking of nanoscale cell surface dynamics. ACS Nano 15, 17613–17622 (2021).
Sánchez, D. et al. Noncontact measurement of the local mechanical properties of living cells using pressure applied via a pipette. Biophys J. 95, 3017–3027 (2008).
Rheinlaender, J. & Schäffer, T. E. Mapping the mechanical stiffness of live cells with the scanning ion conductance microscope. Soft Matter 9, 3230–3236 (2013).
Rheinlaender, J. & Schäffer, T. E. Mapping the creep compliance of living cells with scanning ion conductance microscopy reveals a subcellular correlation between stiffness and fluidity. Nanoscale 11, 6982–6989 (2019).
Wang, Y., Shashishekar, M., Spence, D. M. & Baker, L. A. Subcellular mechanical imaging of erythrocytes with optically correlated scanning ion conductance microscopy. ACS Meas. Sci. Au 5, 345–352 (2025).
Xiao, R., Zhang, Y. & Li, M. Automated high-throughput atomic force microscopy single-cell nanomechanical assay enabled by deep learning-based optical image recognition. Nano Lett. 24, 12323–12332 (2024).
Thomas Chemin, O. et al. Advancing high-throughput cellular atomic force microscopy with automation and artificial intelligence. ACS Nano 19, 5045–5062 (2025).
Shakoor, A., Gao, W., Zhao, L., Jiang, Z. & Sun, D. Advanced tools and methods for single-cell surgery. Microsyst. Nanoeng. 8, 47 (2022).
Moeendarbary, E. et al. The cytoplasm of living cells behaves as a poroelastic material. Nat. Mater. 12, 253–261 (2013).
Stewart, M. P. et al. Wedged AFM-cantilevers for parallel plate cell mechanics. Methods 60, 186–194 (2013).
Gonnermann, C. et al. Quantitating membrane bleb stiffness using AFM force spectroscopy and an optical sideview setup. Integr. Biol. 7, 356–363 (2015).
Chaudhuri, O., Parekh, S. H., Lam, W. A. & Fletcher, D. A. Combined atomic force microscopy and side-view optical imaging for mechanical studies of cells. Nat. Methods 6, 383–387 (2009).
Yang, Y. & Li, M. Side-view optical microscopy-assisted atomic force microscopy for thickness-dependent nanobiomechanics. Nanoscale Adv. 6, 3306–3319 (2024).
Kim, J., Koo, B.-K. & Knoblich, J. A. Human organoids: model systems for human biology and medicine. Nat. Rev. Mol. Cell Biol. 21, 571–584 (2020).
Verstegen, M. M. A. et al. Clinical applications of human organoids. Nat. Med. 31, 409–421 (2025).
Guillaume-Gentil, O. et al. Force-controlled manipulation of single cells: from AFM to FluidFM. Trends Biotechnol. 32, 381–388 (2014).
Acknowledgements
The authors thank all the doctoral students and post-doctoral fellows who invaluably contributed to the progress of the FluidFM. The authors thank D. Ossola, P. Dörig, P. Behr and M. Gabi (Cytosurge AG, CH), D. Bijl (Smarttip BV, NL) as well as P. Frederix, C. Bippes and M. Portalupi (Nanosurf AG, CH) for their constant support. T.Z. is indebted to J. Vörös (ETH Zurich) for his generous continuous trust. The development of the FluidFM and its applications has been enabled by several grants of the Swiss KTI-CTI agency (now Innosuisse) to T.Z. and J.A.V., grants from the Swiss National Science Foundation to T.Z., and a European Research Council Advanced Grant (number 883077) as well as funding from the Swiss State Secretariat for Education, Research and Innovation (SERI) to J.A.V. The contribution of R.H. was supported by the Momentum (Lendület) Program of the Hungarian Academy of Sciences and the National Research, Development, and Innovation Fund (NKFIH) of Hungary under grant TKP2021-EGA-04, ADVANCED 153121 and 2024-1.2.10-TÉT-IPARI-IL-2024-00030. The contribution of M.L. was supported by the National Natural Science Foundation of China (no. 62573403) and the Natural Science Foundation of Liaoning Province (no. 2024JH3/50100021). The contribution of G.-y.L. was supported the National Science Foundation of USA (CHE-2304986).
Author information
Authors and Affiliations
Contributions
Introduction (T.Z., O.G.-G., G.P., R.H., G.-y.L., M.L. and J.A.V.); Experimentation (T.Z., O.G.-G., E.S., G.P., R.H., G.-y.L., M.L. and J.A.V.); Results (T.Z., O.G.-G., G.P., R.H., G.-y.L., M.L. and J.A.V.); Applications (T.Z., O.G.-G., G.P., R.H., G.-y.L., M.L. and J.A.V.); Reproducibility and data deposition (T.Z., O.G.-G., G.P., R.H., G.-y.L., M.L. and J.A.V.); Limitations and optimizations (T.Z., O.G.-G., E.S., G.P., R.H., G.-y.L., M.L. and J.A.V.); Outlook (T.Z., O.G.-G., E.S., G.P., R.H., G.-y.L., M.L. and J.A.V.); overview of the Primer (T.Z. and J.A.V.).
Corresponding author
Ethics declarations
Competing interests
E.S. oversees the production of FluidFM probes and is employed by Bruker Nederland BV (NL). The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Methods Primers thanks Ricardo Garcia, Ana Sancho and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zambelli, T., Guillaume-Gentil, O., Sarajlic, E. et al. Fluidic force microscopy. Nat Rev Methods Primers 6, 15 (2026). https://doi.org/10.1038/s43586-025-00463-2
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s43586-025-00463-2