Abstract
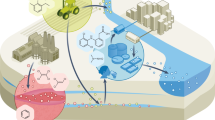
Water quality assessment is exceedingly challenging given the complexity of the anthropogenic chemicals present in the environment. In addition, water treatment is increasingly reliant on chemical oxidants, which transform natural and anthropogenic organic compounds into a wide spectrum of transformation products with unknown toxicities. Existing strategies to evaluate the toxicity of these complex mixtures have so far primarily focused on the application of in vitro assays. Existing in vitro assays provide useful insights into the adverse outcomes for a variety of toxicological endpoints but generally do not provide information about the identities of the toxicant(s) responsible for the observed effect in environmental samples. Advancements in in vitro assays combined with non-targeted analysis show substantial progress in identifying emerging chemicals of concern, albeit with selection biases for analytes that are compatible with sample extraction and preparation approaches. Here we discuss the application of molecular toxicology (in chemico) approaches as a promising complement to in vitro assays to assess water quality and responsible toxicants. These in chemico approaches show particular promise for compounds that are challenging to extract and detect using conventional approaches, such as those that are highly polar, reactive (for example, organic electrophiles) and/or volatile compounds. We structure the discussion of the different in chemico approaches around the molecular initiating event, which is the initial step of the adverse outcome pathway that describes the molecular-level interactions between toxicants and organisms. In chemico approaches that use biomolecules of different complexities to investigate covalent and non-covalent interactions with contaminants are highlighted. This includes in chemico studies focusing on (1) the assessment of individual contaminants, (2) the overall toxicity of samples from laboratory studies or the environment and (3) the identification of toxicants in complex (environmental) mixtures. Major advancements in each of these areas are discussed, and future major research needs are outlined.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Schwarzenbach, R. P. et al. The challenge of micropollutants in aquatic systems. Science 313, 1072–1077 (2006).
Farré, M. L., Pérez, S., Kantiani, L. & Barceló, D. Fate and toxicity of emerging pollutants, their metabolites and transformation products in the aquatic environment. TrAC Trends Anal. Chem. 27, 991–1007 (2008).
Escher, B. I. & Fenner, K. Recent advances in environmental risk assessment of transformation products. Environ. Sci. Technol. 45, 3835–3847 (2011).
Von Gunten, U. Oxidation processes in water treatment: are we on track? Environ. Sci. Technol. 52, 5062–5075 (2018).
Lau, S. S., Forster, A. L., Richardson, S. D. & Mitch, W. A. Disinfection byproduct recovery during extraction and concentration in preparation for chemical analyses or toxicity assays. Environ. Sci. Technol. 55, 14136–14145 (2021).
Muellner, M. G. et al. Haloacetonitriles vs. regulated haloacetic acids: are nitrogen-containing DBPs more toxic? Environ. Sci. Technol. 41, 645–651 (2007).
Schmeisser, S. et al. New approach methodologies in human regulatory toxicology—not if, but how and when! Environ. Int. 178, 108082 (2023).
Dix, D. J. et al. The ToxCast program for prioritizing toxicity testing of environmental chemicals. Toxicol. Sci. 95, 5–12 (2007).
Judson, R. et al. In vitro and modelling approaches to risk assessment from the U.S. Environmental Protection Agency ToxCast Programme. Basic Clin. Pharmacol. Toxicol. 115, 69–76 (2014).
Richard, A. M. et al. ToxCast chemical landscape: paving the road to 21st century toxicology. Chem. Res. Toxicol. 29, 1225–1251 (2016).
Murk, A. J. et al. Detection of estrogenic potency in wastewater and surface water with three in vitro bioassays. Environ. Toxicol. Chem. 21, 16–23 (2002).
Prasse, C., Stalter, D., Schulte-Oehlmann, U., Oehlmann, J. & Ternes, T. A. Spoilt for choice: a critical review on the chemical and biological assessment of current wastewater treatment technologies. Water Res. 87, 237–270 (2015).
Stalter, D., O’Malley, E., von Gunten, U. & Escher, B. I. Fingerprinting the reactive toxicity pathways of 50 drinking water disinfection by-products. Water Res. 91, 19–30 (2016).
König, M. et al. Impact of untreated wastewater on a major European river evaluated with a combination of in vitro bioassays and chemical analysis. Environ. Pollut. 220, 1220–1230 (2017).
Žegura, B., Heath, E., Černoša, A. & Filipič, M. Combination of in vitro bioassays for the determination of cytotoxic and genotoxic potential of wastewater, surface water and drinking water samples. Chemosphere 75, 1453–1460 (2009).
Escher, B. I. et al. Benchmarking organic micropollutants in wastewater, recycled water and drinking water with in vitro bioassays. Environ. Sci. Technol. 48, 1940–1956 (2014).
Leusch, F. D. L. et al. Analysis of endocrine activity in drinking water, surface water and treated wastewater from six countries. Water Res. 139, 10–18 (2018).
Muschket, M. et al. Identification of unknown antiandrogenic compounds in surface waters by Effect-Directed Analysis (EDA) using a parallel fractionation approach. Environ. Sci. Technol. 52, 288–297 (2018).
Morandi, G. D. et al. Effects-directed analysis of dissolved organic compounds in oil sands process-affected water. Environ. Sci. Technol. 49, 12395–12404 (2015).
Brack, W. Effect-directed analysis: a promising tool for the identification of organic toxicants in complex mixtures? Anal. Bioanal.Chem. 377, 397–407 (2003).
Tian, Z. et al. A ubiquitous tire rubber-derived chemical induces acute mortality in coho salmon. Science 371, 185–189 (2021).
Brack, W. et al. Effect-directed analysis supporting monitoring of aquatic environments—an in-depth overview. Sci. Total Environ. 544, 1073–1118 (2016).
Abbas, A. et al. What you extract is what you see: optimising the preparation of water and wastewater samples for in vitro bioassays. Water Res. 152, 47–60 (2019).
US EPA. Alternative Test Methods and Strategies to Reduce Vertebrate Animal Testing https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/alternative-test-methods-and-strategies-reduce#:~:text=Examples%20of%20NAMs%20would%20be,for%20computer%2Ddriven%20predictive%20tools (Environmental Protection Agency, 2024).
Ankley, G. T. et al. Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ. Toxicol. Chem. 29, 730–741 (2010).
Villeneuve, D. L. et al. Adverse Outcome Pathway (AOP) development I: strategies and principles. Toxicol. Sci. 142, 312–320 (2014).
Gerberick, G. F. Development of a peptide reactivity assay for screening contact allergens. Toxicol. Sci. 81, 332–343 (2004).
Natsch, A. Integrated skin sensitization assessment based on OECD methods (I): deriving a point of departure for risk assessment. ALTEX 39, 636–646 (2022).
Dik, S., Rorije, E., Schwillens, P., Van Loveren, H. & Ezendam, J. Can the direct peptide reactivity assay be used for the identification of respiratory sensitization potential of chemicals? Toxicol. Sci. 153, 361–371 (2016).
Lalko, J. F. et al. The direct peptide reactivity assay: selectivity of chemical respiratory allergens. Toxicol. Sci. 129, 421–431 (2012).
Marcelis, Q., Deconinck, E., Rogiers, V., Vanhaecke, T. & Desmedt, B. Applicability of the DPRA on mixture testing: challenges and opportunities. Arch. Toxicol. 97, 2453–2461 (2023).
Jaria, G., Calisto, V., Otero, M. & Esteves, V. I. Monitoring pharmaceuticals in the aquatic environment using enzyme-linked immunosorbent assay (ELISA)—a practical overview. Anal. Bioanal. Chem. 412, 3983–4008 (2020).
Silva, C. P., Carvalho, T., Schneider, R. J., Esteves, V. I. & Lima, D. L. D. ELISA as an effective tool to determine spatial and seasonal occurrence of emerging contaminants in the aquatic environment. Anal. Methods 12, 2517–2526 (2020).
Patel, D., Huma, Z. E. & Duncan, D. Reversible covalent inhibition─desired covalent adduct formation by mass action. ACS Chem. Biol. 19, 824–838 (2024).
Boike, L., Henning, N. J. & Nomura, D. K. Advances in covalent drug discovery. Nat. Rev. Drug Discov. 21, 881–898 (2022).
Pals, J. A., Wagner, E. D. & Plewa, M. J. Energy of the lowest unoccupied molecular orbital, thiol reactivity, and toxicity of three monobrominated water disinfection byproducts. Environ. Sci. Technol. 50, 3215–3221 (2016).
Pals, J. A., Wagner, E. D., Plewa, M. J., Xia, M. & Attene-Ramos, M. S. Monohalogenated acetamide-induced cellular stress and genotoxicity are related to electrophilic softness and thiol/thiolate reactivity. J. Environ. Sci. 58, 224–230 (2017).
Zhong, C., Zhao, H., Cao, H. & Huang, C.-H. Fast coupling and detoxification of aqueous halobenzoquinones by extracellular nucleophiles: the relationship among structures, pathways and toxicity. Chem. Eng. J. 438, 135525 (2022).
Dawson, D. A., Jeyaratnam, J., Mooneyham, T., Pöch, G. & Schultz, T. W. Mixture toxicity of SN2-reactive soft electrophiles: 1. Evaluation of mixtures containing α-halogenated acetonitriles. Arch. Environ. Contam. Toxicol. 59, 532–541 (2010).
Yeung, K., Xie, L., Nair, P. & Peng, H. Haloacetonitriles induce structure-related cellular toxicity through distinct proteome thiol reaction mechanisms. ACS Environ. Au 5, 101–113 (2025).
Lin, E. L. C. & Guion, C. W. Interaction of haloacetonitriles with glutathione and glutathione-S-transferase. Biochem. Pharmacol. 38, 685–688 (1989).
Hong, H. et al. Cytotoxicity of nitrogenous disinfection byproducts: a combined experimental and computational study. Sci. Total Environ. 856, 159273 (2023).
Böhme, A., Thaens, D., Paschke, A. & Schüürmann, G. Kinetic glutathione chemoassay to quantify thiol reactivity of organic electrophiles—application to α,β-unsaturated ketones, acrylates and propiolates. Chem. Res. Toxicol. 22, 742–750 (2009).
Slawik, C., Rickmeyer, C., Brehm, M., Böhme, A. & Schüürmann, G. Glutathione adduct patterns of Michael-acceptor carbonyls. Environ. Sci. Technol. 51, 4018–4026 (2017).
Li, Y., Jongberg, S., Andersen, M. L., Davies, M. J. & Lund, M. N. Quinone-induced protein modifications: kinetic preference for reaction of 1,2-benzoquinones with thiol groups in proteins. Free Radic. Biol. Med. 97, 148–157 (2016).
Shu, N., Lorentzen, L. G. & Davies, M. J. Reaction of quinones with proteins: kinetics of adduct formation, effects on enzymatic activity and protein structure, and potential reversibility of modifications. Free Radic. Biol. Med. 137, 169–180 (2019).
Prasse, C., Ford, B., Nomura, D. K. & Sedlak, D. L. Unexpected transformation of dissolved phenols to toxic dicarbonyls by hydroxyl radicals and UV light. Proc. Natl. Acad. Sci. USA 115, 2311–2316 (2018).
Hall, D. R., Yeung, K. & Peng, H. Monohaloacetic acids and monohaloacetamides attack distinct cellular proteome thiols. Environ. Sci. Technol. 54, 15191–15201 (2020).
Hall, D. R., Gauthier, J. & Peng, H. Querying the in vitro proteome cysteine reactivity of 8:2 fluorotelomer acrylate. Environ. Sci. Technol. 57, 13015–13024 (2023).
Enoch, S. J. & Cronin, M. T. D. A review of the electrophilic reaction chemistry involved in covalent DNA binding. Crit. Rev. Toxicol. 40, 728–748 (2010).
Nguyen, T. N. T., Bertagnolli, A. D., Villalta, P. W., Bühlmann, P. & Sturla, S. J. Characterization of a deoxyguanosine adduct of tetrachlorobenzoquinone: dichlorobenzoquinone-1, N2-etheno-2′-deoxyguanosine. Chem. Res. Toxicol. 18, 1770–1776 (2005).
Vaidyanathan, V. G., Villalta, P. W. & Sturla, S. J. Nucleobase-dependent reactivity of a quinone metabolite of pentachlorophenol. Chem. Res. Toxicol. 20, 913–919 (2007).
Jia, S., Zhu, B.-Z. & Guo, L.-H. Detection and mechanistic investigation of halogenated benzoquinone induced DNA damage by photoelectrochemical DNA sensor. Anal. Bioanal. Chem. 397, 2395–2400 (2010).
Gómez-Bombarelli, R. et al. DNA-damaging disinfection byproducts: alkylation mechanism of mutagenic mucohalic acids. Environ. Sci. Technol. 45, 9009–9016 (2011).
Munter, T., Le Curieux, F., Sjöholm, R. & Kronberg, L. Reaction of the potent bacterial mutagen 3-chloro-4-(dichloromethyl)-5-hydroxy-2(5H)-furanone (MX) with 2′-deoxyadenosine and calf thymus DNA: identification of fluorescent propenoformyl derivatives. Chem. Res. Toxicol. 11, 226–233 (1998).
Munter, T., Le Curieux, F., Sjöholm, R. & Kronberg, L. Identification of an ethenoformyl adduct formed in the reaction of the potent bacterial mutagen 3-chloro-4-(dichloromethyl)-5-hydroxy-2(5H)-furanone with guanosine. Chem. Res. Toxicol. 12, 46–52 (1999).
Le Curieux, F., Munter, T. & Kronberg, L. Identification of adenine adducts formed in reaction of calf thymus DNA with mutagenic chlorohydroxyfuranones found in drinking water. Chem. Res. Toxicol. 10, 1180–1185 (1997).
Dong, S., Masalha, N., Plewa, M. J. & Nguyen, T. H. Toxicity of wastewater with elevated bromide and iodide after chlorination, chloramination or ozonation disinfection. Environ. Sci. Technol. 51, 9297–9304 (2017).
Dong, S., Page, M. A., Wagner, E. D. & Plewa, M. J. Thiol reactivity analyses to predict mammalian cell cytotoxicity of water samples. Environ. Sci. Technol. 52, 8822–8829 (2018).
Dong, S. et al. Toxicological comparison of water, wastewaters and processed wastewaters. Environ. Sci. Technol. 53, 9139–9147 (2019).
Prasse, C., Von Gunten, U. & Sedlak, D. L. Chlorination of phenols revisited: unexpected formation of α,β-unsaturated C4-dicarbonyl ring cleavage products. Environ. Sci. Technol. 54, 826–834 (2020).
Zhang, Z. & Prasse, C. Chlorination of para-substituted phenols: formation of α,β-unsaturated C4-dialdehydes and C4-dicarboxylic acids. J. Environ. Sci. 117, 197–208 (2022).
Zoumpouli, G. A., Zhang, Z., Wenk, J. & Prasse, C. Aqueous ozonation of furans: kinetics and transformation mechanisms leading to the formation of α,β-unsaturated dicarbonyl compounds. Water Res. 203, 117487 (2021).
Prasse, C. Reactivity-directed analysis—a novel approach for the identification of toxic organic electrophiles in drinking water. Environ. Sci. Process. Impacts 23, 48–65 (2021).
Radke, M. J., Cresswell, S. L. & Leusch, F. D. L. Combining non-targeted high resolution mass spectrometry with effect-directed analysis to identify contaminants of emerging concern in the field of ecotoxicology: a systematic quantitative literature review. Sci. Total Environ. 972, 179122 (2025).
Tian, Z., McMinn, M. H. & Fang, M. Effect-directed analysis and beyond: how to find causal environmental toxicants. Exposome 3, osad002 (2023).
Timilsina, A., Lokesh, S., Shahriar, A., Numan, T. & Yang, Y. Quantification of quinones in environmental media by chemical tagging with cysteine-containing peptides coupled to size exclusionary separation. Anal. Chem. 95, 12575–12579 (2023).
Yeung, K. et al. Thiol reactome: a nontargeted strategy to precisely identify thiol reactive drinking water disinfection byproducts. Environ. Sci. Technol. 57, 18722–18734 (2023).
Xu, X. et al. Applications of human and bovine serum albumins in biomedical engineering: a review. Int. J. Biol. Macromol. 253, 126914 (2023).
Ju, P. et al. Probing the toxic interactions between polyvinyl chloride microplastics and human serum albumin by multispectroscopic techniques. Sci. Total Environ. 734, 139219 (2020).
Chi, Z. & Liu, R. Phenotypic characterization of the binding of tetracycline to human serum albumin. Biomacromolecules 12, 203–209 (2011).
Abou-Zied, O. K. & Al-Shihi, O. I. K. Characterization of subdomain IIA binding site of human serum albumin in its native, unfolded, and refolded states using small molecular probes. J. Am. Chem. Soc. 130, 10793–10801 (2008).
Xiang, H. et al. Study of conformational and functional changes caused by binding of environmental pollutant tonalide to human serum albumin. Chemosphere 270, 129431 (2021).
Zargar, S. & Wani, T. A. Exploring the binding mechanism and adverse toxic effects of persistent organic pollutant (dicofol) to human serum albumin: a biophysical, biochemical and computational approach. Chem. Biol. Interact. 350, 109707 (2021).
Ahmad, M. I., Potshangbam, A. M., Javed, M. & Ahmad, M. Studies on conformational changes induced by binding of pendimethalin with human serum albumin. Chemosphere 243, 125270 (2020).
Qian, Y., Zhou, X., Chen, J. & Zhang, Y. Binding of bezafibrate to human serum albumin: insight into the non-covalent interaction of an emerging contaminant with biomacromolecules. Molecules 17, 6821–6831 (2012).
Chen, J., Zhou, X., Zhang, Y., Qian, Y. & Gao, H. Interactions of acidic pharmaceuticals with human serum albumin: insights into the molecular toxicity of emerging pollutants. Amino Acids 43, 1419–1429 (2012).
Hou, C. et al. Study of modeling and optimization for predicting the acute toxicity of carbamate pesticides using the binding information with carrier protein. Spectrochim. Acta A 273, 121038 (2022).
Mátyus, L., Szöllősi, J. & Jenei, A. Steady-state fluorescence quenching applications for studying protein structure and dynamics. J. Photochem. Photobiol. B 83, 223–236 (2006).
Tanwar, A. S., Parui, R., Garai, R., Chanu, M. A. & Iyer, P. K. Dual ‘static and dynamic’ fluorescence quenching mechanisms based detection of TNT via a cationic conjugated polymer. ACS Meas. Sci. Au 2, 23–30 (2022).
Ross, P. D. & Subramanian, S. Thermodynamics of protein association reactions: forces contributing to stability. Biochemistry 20, 3096–3102 (1981).
Moro, G. et al. Investigation of the interaction between human serum albumin and branched short-chain perfluoroalkyl compounds. Chem. Res. Toxicol. 35, 2049–2058 (2022).
Poureshghi, F., Ghandforoushan, P., Safarnejad, A. & Soltani, S. Interaction of an antiepileptic drug, lamotrigine with human serum albumin (HSA): application of spectroscopic techniques and molecular modeling methods. J. Photochem. Photobiol. B 166, 187–192 (2017).
Van De Weert, M. & Schönbeck, C. Ligand binding to proteins—when flawed fluorescence quenching methodology and interpretation become the new norm. Eur. J. Pharm. Sci. 203, 106930 (2024).
Qin, W., Escher, B. I., Huchthausen, J., Fu, Q. & Henneberger, L. Species difference? Bovine, trout and human plasma protein binding of per- and polyfluoroalkyl substances. Environ. Sci. Technol. 58, 9954–9966 (2024).
Ebert, A., Allendorf, F., Berger, U., Goss, K.-U. & Ulrich, N. Membrane/water partitioning and permeabilities of perfluoroalkyl acids and four of their alternatives and the effects on toxicokinetic behavior. Environ. Sci. Technol. 54, 5051–5061 (2020).
Jia, Y. et al. Insights into the competitive mechanisms of per- and polyfluoroalkyl substances partition in liver and blood. Environ. Sci. Technol. 56, 6192–6200 (2022).
Fischer, F. C. et al. Binding of per- and polyfluoroalkyl substances (PFAS) to serum proteins: implications for toxicokinetics in humans. Environ. Sci. Technol. 58, 1055–1063 (2024).
Allendorf, F., Berger, U., Goss, K.-U. & Ulrich, N. Partition coefficients of four perfluoroalkyl acid alternatives between bovine serum albumin (BSA) and water in comparison to ten classical perfluoroalkyl acids. Environ. Sci. Process. Impacts 21, 1852–1863 (2019).
Crisalli, A. M., Cai, A. & Cho, B. P. Probing the interactions of perfluorocarboxylic acids of various chain lengths with human serum albumin: calorimetric and spectroscopic investigations. Chem. Res. Toxicol. 36, 703–713 (2023).
Greenfield, N. J. Using circular dichroism spectra to estimate protein secondary structure. Nat. Protoc. 1, 2876–2890 (2006).
Zhang, Y.-Z. et al. Fluorescence study on the interaction of bovine serum albumin with P-aminoazobenzene. J. Fluoresc. 18, 109–118 (2008).
Partridge, A. W., Therien, A. G. & Deber, C. M. Polar mutations in membrane proteins as a biophysical basis for disease. Biopolymers 66, 350–358 (2002).
Partridge, A. W., Therien, A. G. & Deber, C. M. Missense mutations in transmembrane domains of proteins: phenotypic propensity of polar residues for human disease. Proteins 54, 648–656 (2004).
Sekula, B., Zielinski, K. & Bujacz, A. Crystallographic studies of the complexes of bovine and equine serum albumin with 3,5-diiodosalicylic acid. Int. J. Biol. Macromol. 60, 316–324 (2013).
Maier, R., Fries, M. R., Buchholz, C., Zhang, F. & Schreiber, F. Human versus bovine serum albumin: a subtle difference in hydrophobicity leads to large differences in bulk and interface behavior. Cryst. Growth Des. 21, 5451–5459 (2021).
Huang, B. X., Kim, H.-Y. & Dass, C. Probing three-dimensional structure of bovine serum albumin by chemical cross-linking and mass spectrometry. J. Am. Soc. Mass Spectrom. 15, 1237–1247 (2004).
Ketrat, S., Japrung, D. & Pongprayoon, P. Exploring how structural and dynamic properties of bovine and canine serum albumins differ from human serum albumin. J. Mol. Graph. Model. 98, 107601 (2020).
Bischel, H. N., MacManus‐Spencer, L. A., Zhang, C. & Luthy, R. G. Strong associations of short‐chain perfluoroalkyl acids with serum albumin and investigation of binding mechanisms. Environ. Toxicol. Chem. 30, 2423–2430 (2011).
Wang, Y.-Q., Zhang, H.-M., Cao, J. & Tang, B.-P. Binding of a new bisphenol analogue, bisphenol S to bovine serum albumin and calf thymus DNA. J. Photochem. Photobiol. B 138, 182–190 (2014).
Chi, Z., Liu, R., Teng, Y., Fang, X. & Gao, C. Binding of oxytetracycline to bovine serum albumin: spectroscopic and molecular modeling investigations. J. Agric. Food Chem. 58, 10262–10269 (2010).
Yadav, A., Vuković, L. & Narayan, M. An atomic and molecular insight into how PFOA reduces α-helicity, compromises substrate binding, and creates binding pockets in a model globular protein. J. Am. Chem. Soc. 146, 12766–12777 (2024).
Sheng, N., Li, J., Liu, H., Zhang, A. & Dai, J. Interaction of perfluoroalkyl acids with human liver fatty acid-binding protein. Arch. Toxicol. 90, 217–227 (2016).
Chen, Y. et al. Overall and individual associations between per- and polyfluoroalkyl substances and liver function indices and the metabolic mechanism. Environ. Int. 183, 108405 (2024).
Zhang, L., Ren, X.-M. & Guo, L.-H. Structure-based investigation on the interaction of perfluorinated compounds with human liver fatty acid binding protein. Environ. Sci. Technol. 47, 11293–11301 (2013).
Song, Y., Sun, K. & Liu, R. An exploration of the interaction mechanism of Direct Red 80 with α‐amylase at the molecular level. J. Mol. Recognit. 34, e2883 (2021).
Liu, Y. & Liu, R. The interaction of α-chymotrypsin with one persistent organic pollutant (dicofol): spectroscope and molecular modeling identification. Food Chem. Toxicol. 50, 3298–3305 (2012).
Chi, Z., Liu, R. & Zhang, H. Noncovalent interaction of oxytetracycline with the enzyme trypsin. Biomacromolecules 11, 2454–2459 (2010).
Anichina, J., Zhao, Y., Hrudey, S. E., Le, X. C. & Li, X.-F. Electrospray ionization mass spectrometry characterization of interactions of newly identified water disinfection byproducts halobenzoquinones with oligodeoxynucleotides. Environ. Sci. Technol. 44, 9557–9563 (2010).
Chen, Y.-H. et al. Synergetic effects of novel aromatic brominated and chlorinated disinfection byproducts on Vibrio qinghaiensis sp.-Q67. Environ. Pollut. 250, 375–385 (2019).
Richardson, S., Plewa, M., Wagner, E., Schoeny, R. & Demarini, D. Occurrence, genotoxicity and carcinogenicity of regulated and emerging disinfection by-products in drinking water: a review and roadmap for research. Mutat. Res. 636, 178–242 (2007).
Li, G., Tian, C., Karanfil, T. & Liu, C. Comparative formation of chlorinated and brominated disinfection byproducts from chlorination and bromination of amino acids. Chemosphere 349, 140985 (2024).
Anichina, J., Zhao, Y., Hrudey, S. E., Schreiber, A. & Li, X.-F. Electrospray ionization tandem mass spectrometry analysis of the reactivity of structurally related bromo-methyl-benzoquinones toward oligonucleotides. Anal. Chem. 83, 8145–8151 (2011).
Tomkinson, A. E., Lasko, D. D., Daly, G. & Lindahl, T. Mammalian DNA ligases. Catalytic domain and size of DNA ligase I. J. Biol. Chem. 265, 12611–12617 (1990).
Tu, N. et al. Quantitative structure-toxicity relationships of halobenzoquinone isomers on DNA reactivity and genotoxicity. Chemosphere 309, 136763 (2022).
Wen, H. et al. Noncovalent tagging for identifying unknown contaminants of specific bioactivity in environmental water. Anal. Chem. 95, 15851–15855 (2023).
Mann, M. M., Tang, J. D. & Berger, B. W. Engineering human liver fatty acid binding protein for detection of poly‐ and perfluoroalkyl substances. Biotechnol. Bioeng. 119, 513–522 (2022).
Zhang, Q., Dong, X., Lu, J., Song, J. & Wang, Y. Chemoproteomic approach toward probing the interactomes of perfluoroalkyl substances. Anal. Chem. 93, 9634–9639 (2021).
Gong, Y., Yang, D., Barrett, H., Sun, J. & Peng, H. Building the environmental Chemical-Protein Interaction Network (eCPIN): an exposome-wide strategy for bioactive chemical contaminant identification. Environ. Sci. Technol. 57, 3486–3495 (2023).
Yang, D. et al. Nontarget screening of per- and polyfluoroalkyl substances binding to human liver fatty acid binding protein. Environ. Sci. Technol. 54, 5676–5686 (2020).
Reinemann, C., Freiin Von Fritsch, U., Rudolph, S. & Strehlitz, B. Generation and characterization of quinolone-specific DNA aptamers suitable for water monitoring. Biosens. Bioelectron. 77, 1039–1047 (2016).
Cao, J. et al. Parallel detection of mixed pesticides based on dual quantum dot/porous silicon optical biosensors. IEEE Sensors J. 24, 38596–38604 (2024).
Park, J., Yang, K.-A., Choi, Y. & Choe, J. K. Novel ssDNA aptamer-based fluorescence sensor for perfluorooctanoic acid detection in water. Environ. Int. 158, 107000 (2022).
Wen, K., Meng, X., Lara, K. & Lin, Q. Cost-effective evaluation of aptamer candidates in SELEX-based aptamer isolation. Talanta 275, 126103 (2024).
Imashimizu, M., Takahashi, M., Amano, R. & Nakamura, Y. Single-round isolation of diverse RNA aptamers from a random sequence pool. Biol. Methods Protoc. 3, bpy004 (2018).
Gulde, R. et al. Oxidation of 51 micropollutants during drinking water ozonation: formation of transformation products and their fate during biological post-filtration. Water Res. 207, 117812 (2021).
Lim, S., Shi, J. L., Von Gunten, U. & McCurry, D. L. Ozonation of organic compounds in water and wastewater: a critical review. Water Res. 213, 118053 (2022).
Grace, D. N., Newmeyer, M. N. & Prasse, C. Solid-Phase Reactivity-Directed Extraction (SPREx): an alternative approach for simultaneous extraction, identification and prioritization of toxic electrophiles produced in water treatment applications. ACS Environ. Au 4, 317–332 (2024).
Johnson, M., Finlayson, K., Van De Merwe, J. P. & Leusch, F. D. L. Adaption and application of cell-based bioassays to whole-water samples. Chemosphere 361, 142572 (2024).
Schirmer, K., Dayeh, V. R., Bopp, S., Russold, S. & Bols, N. C. Applying whole water samples to cell bioassays for detecting dioxin-like compounds at contaminated sites. Toxicology 205, 211–221 (2004).
Hosaka, S., Honda, T., Lee, S. H. & Oe, T. Biomimetic trapping cocktail to screen reactive metabolites: use of an amino acid and DNA motif mixture as light/heavy isotope pairs differing in mass shift. Anal. Bioanal. Chem. 410, 3847–3857 (2018).
Manasfi, T., Houska, J., Gebhardt, I. & von Gunten, U. Formation of carbonyl compounds during ozonation of lake water and wastewater: development of a non-target screening method and quantification of target compounds. Water Res. 237, 119751 (2023).
Houska, J., Manasfi, T., Gebhardt, I. & von Gunten, U. Ozonation of lake water and wastewater: identification of carbonous and nitrogenous carbonyl-containing oxidation byproducts by non-target screening. Water Res. 232, 119484 (2022).
Liu, G. et al. Probing protein-protein interactions with label-free mass spectrometry quantification in combination with affinity purification by spin-tip affinity columns. Anal. Chem. 92, 3913–3922 (2020).
Cho, N. et al. Identification of novel glutathione adducts of benzbromarone in human liver microsomes. Drug Metab. Pharmacokinet. 32, 46–52 (2017).
Kalgutkar, A. S. et al. Metabolic activation of the nontricyclic antidepressant trazodone to electrophilic quinone-imine and epoxide intermediates in human liver microsomes and recombinant P4503A4. Chem. Biol. Interact. 155, 10–20 (2005).
Tian, M., Peng, Y. & Zheng, J. Metabolic activation and hepatotoxicity of furan-containing compounds. Drug Metab. Dispos. 50, 655–670 (2022).
Ahlfors, S. R., Sterner, O. & Hansson, C. Reactivity of contact allergenic haptens to amino acid residues in a model carrier peptide, and characterization of formed peptide-hapten adducts1. Skin Pharmacol. Physiol. 16, 59–68 (2003).
Medina-Cleghorn, D. et al. Mapping proteome-wide targets of environmental chemicals using reactivity-based chemoproteomic platforms. Chem. Biol. 22, 1394–1405 (2015).
Ferraro, P. J. & Prasse, C. Reimagining safe drinking water on the basis of twenty-first-century science. Nat. Sustain. 4, 1032–1037 (2021).
Ankley, G. T. & Edwards, S. W. The adverse outcome pathway: a multifaceted framework supporting 21st century toxicology. Curr. Opin. Toxicol. 9, 1–7 (2018).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Poirier, M. C. Chemical-induced DNA damage and human cancer risk. Nat. Rev. Cancer 4, 630–637 (2004).
Smith, M. T. et al. Key characteristics of carcinogens as a basis for organizing data on mechanisms of carcinogenesis. Environ. Health Perspect. 124, 713–721 (2016).
Rappaport, S. M., Li, H., Grigoryan, H., Funk, W. E. & Williams, E. R. Adductomics: characterizing exposures to reactive electrophiles. Toxicol. Lett. 213, 83–90 (2012).
Acknowledgements
This study was supported by the US National Science Foundation (CAREER award 2143152) and the US Environmental Protection Agency (R840605). D.N.G. was supported by a National Science Foundation Graduate Research Fellowship under grant no. DGE2139757, and A.R. acknowledges financial support from Johns Hopkins University through the Vivien Thomas Scholars Initiative.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Water thanks Peng Hui and Dan Villeneuve for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Grace, D.N., Rorie, A. & Prasse, C. In chemico toxicity approaches to assess, identify and prioritize contaminants in water. Nat Water 3, 854–866 (2025). https://doi.org/10.1038/s44221-025-00468-x
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44221-025-00468-x