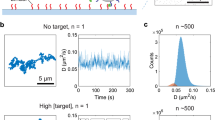
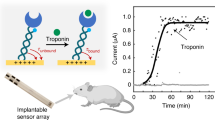
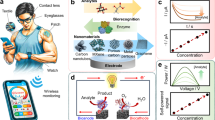
Abstract
Body-based biomolecular sensing systems, including wearable, implantable and consumable sensors allow comprehensive health-related monitoring. Glucose sensors have long dominated wearable bioanalysis applications owing to their robust continuous detection of glucose, which has not yet been achieved for other biomarkers. However, access to diverse biological fluids and the development of reagentless sensing approaches may enable the design of body-based sensing systems for various analytes. Importantly, enhancing the selectivity and sensitivity of biomolecular sensors is essential for biomarker detection in complex physiological conditions. In this Review, we discuss approaches for the signal amplification of biomolecular sensors, including techniques to overcome Debye and mass transport limitations, and selectivity improvement, such as the integration of artificial affinity recognition elements. We highlight reagentless sensing approaches that can enable sequential real-time measurements, for example, the implementation of thin-film transistors in wearable devices. In addition to sensor construction, careful consideration of physical, psychological and security concerns related to body-based sensor integration is required to ensure that the transition from the laboratory to the human body is as seamless as possible.
Key points
-
Glucose sensors traditionally dominate the commercial sensing market, but sensors for alternative analytes could advance personalized health care in coming decades.
-
Body-based biomolecular sensors, including wearable, implantable and ingestible sensors, provide simple and continuous access to user biomolecular data through various biological fluids.
-
Continuous monitoring requires kinetically favourable receptors and sensing mechanisms capable of detecting analytes without user intervention.
-
In addition to sensor efficacy, body-based systems require careful consideration of physical, psychological and security concerns related to device use and data handling.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Ji, S. et al. Matching the kinetics of natural enzymes with a single-atom iron nanozyme. Nat. Catal. 4, 407–417 (2021).
Armstrong, J. A. Urinalysis in Western culture: a brief history. Kidney Int. 71, 384–387 (2007).
Karamanou, M., Protogerou, A., Tsoucalas, G., Androutsos, G. & Poulakou-Rebelakou, E. Milestones in the history of diabetes mellitus: the main contributors. World J. Diabetes 7, 1–7 (2016).
Thomas, M. C., Jandeleit-Dahm, K. & Bonnet, F. Beyond glycosuria: exploring the intrarenal effects of SGLT-2 inhibition in diabetes. Diabetes Metab. 40, S17–S22 (2014).
Guthrie, D. W. & Humphreys, S. S. Diabetes urine testing: an historical perspective. Diabetes Educ. 14, 521–525 (1988).
Clark, L. C. Jr & Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. NY Acad. Sci. 102, 29–45 (1962). This article describes the first electrochemical sensing platform that incorporates a biorecognition element to provide selectivity for an analyte.
Clark, L. C., Kaplan, S., Matthews, E. C., Edwards, F. K. & Helmsworth, J. A. Monitor and control of blood oxygen tension and pH during total body perfusion. J. Thorac. Surg. 36, 488–496 (1958).
Free, A. H., Adams, E. C., Kercher, M. L., Free, H. M. & Cook, M. H. Simple specific test for urine glucose. Clin. Chem. 3, 163–168 (1957). This article describes the first time a biorecognition element was incorporated into a sensing mechanism, despite this feat being usually misattributed to Clark and Lyons (1962).
Mazzaferri, E. L., Skillman, T. G., Lanese, R. R. & Keller, M. P. Use of test strips with colour meter to measure blood-glucose. Lancet 295, 331–333 (1970).
Chua, K. S. & Tan, I. K. Plasma glucose measurement with the Yellow Springs glucose analyzer. Clin. Chem. 24, 150–152 (1978).
Clarke, S. F. & Foster, J. R. A history of blood glucose meters and their role in self-monitoring of diabetes mellitus. Br. J. Biomed. Sci. 69, 83–93 (2012).
Matthews, D. R. et al. Pen-sized digital 30-second blood glucose meter. Lancet 329, 778–779 (1987).
Ginsberg, B. H. The FDA panel advises approval of the first continuous glucose sensor. Diabetes Technol. Ther. 1, 203–204 (1999).
Kelley, S. O. et al. Advancing the speed, sensitivity and accuracy of biomolecular detection using multi-length-scale engineering. Nat. Nanotechnol. 9, 969–980 (2014).
Sero, J. E. & Stevens, M. M. Nanoneedle-based materials for intracellular studies. In Bio-Nanomedicine For Cancer Therapy 191–219 (Springer, 2021).
Chiappini, C. et al. Mapping local cytosolic enzymatic activity in human esophageal mucosa with porous silicon nanoneedles. Adv. Mater. 27, 5147–5152 (2015).
Cao, Y. et al. Nondestructive nanostraw intracellular sampling for longitudinal cell monitoring. Proc. Natl Acad. Sci. USA 114, E1866–E1874 (2017).
Siciliano, V. et al. Engineering modular intracellular protein sensor–actuator devices. Nat. Commun. 9, 1881 (2018).
Poste, G. Bring on the biomarkers. Nature 469, 156–157 (2011).
Yang, Y. et al. A laser-engraved wearable sensor for sensitive detection of uric acid and tyrosine in sweat. Nat. Biotechnol. 38, 217–224 (2020).
Weinhofer, I. et al. Neurofilament light chain as a potential biomarker for monitoring neurodegeneration in X-linked adrenoleukodystrophy. Nat. Commun. 12, 1816 (2021).
Kwong, G. A. et al. Synthetic biomarkers: a twenty-first century path to early cancer detection. Nat. Rev. Cancer 21, 655–668 (2021). This review describes the development of synthetic biomarkers, including their function at the physiological level, their design and how they advance biomolecular sensing.
Nishihara, T. et al. Beta-galactosidase-responsive synthetic biomarker for targeted tumor detection. Chem. Commun. 54, 11745–11748 (2018).
Chan, L. W. et al. Engineering synthetic breath biomarkers for respiratory disease. Nat. Nanotechnol. 15, 792–800 (2020).
Aalipour, A. et al. Engineered immune cells as highly sensitive cancer diagnostics. Nat. Biotechnol. 37, 531–539 (2019).
Chen, J. et al. Glucose-oxidase like catalytic mechanism of noble metal nanozymes. Nat. Commun. 12, 3375 (2021).
Liu, S. et al. Metal–organic frameworks and their derivatives as signal amplification elements for electrochemical sensing. Coord. Chem. Rev. 424, 213520 (2020).
Huang, Y., Ren, J. & Qu, X. Nanozymes: classification, catalytic mechanisms, activity regulation, and applications. Chem. Rev. 119, 4357–4412 (2019).
Hu, W.-C. et al. Ultrasensitive detection of bacteria using a 2D MOF nanozyme-amplified electrochemical detector. Anal. Chem. 93, 8544–8552 (2021).
Huang, L., Chen, J., Gan, L., Wang, J. & Dong, S. Single-atom nanozymes. Sci. Adv. 5, eaav5490 (2019).
Jiao, L. et al. Single-atom catalysts boost signal amplification for biosensing. Chem. Soc. Rev. 50, 750–765 (2021).
Wang, Z. et al. Accelerated discovery of superoxide-dismutase nanozymes via high-throughput computational screening. Nat. Commun. 12, 6866 (2021).
Zhong, M. et al. Accelerated discovery of CO2 electrocatalysts using active machine learning. Nature 581, 178–183 (2020).
McConnell, E. M. et al. Biosensing with DNAzymes. Chem. Soc. Rev. 50, 8954–8994 (2021).
Borggräfe, J. et al. Time-resolved structural analysis of an RNA-cleaving DNA catalyst. Nature 601, 144–149 (2022).
Liu, K., Lat, P. K., Yu, H.-Z. & Sen, D. CLICK-17, a DNA enzyme that harnesses ultra-low concentrations of either Cu+ or Cu2+ to catalyze the azide–alkyne ‘click’ reaction in water. Nucleic Acids Res. 48, 7356–7370 (2020).
Liu, M. et al. Programming a topologically constrained DNA nanostructure into a sensor. Nat. Commun. 7, 12074 (2016).
Kang, D.-K. et al. Rapid detection of single bacteria in unprocessed blood using integrated comprehensive droplet digital detection. Nat. Commun. 5, 5427 (2014).
Shen, J., Liu, G., Han, Y. & Jin, W. Artificial channels for confined mass transport at the sub-nanometre scale. Nat. Rev. Mater. 6, 294–312 (2021).
de Angelis, F. et al. Breaking the diffusion limit with super-hydrophobic delivery of molecules to plasmonic nanofocusing SERS structures. Nat. Photon. 5, 682–687 (2011).
Morales-Narváez, E., Guix, M., Medina-Sánchez, M., Mayorga-Martinez, C. C. & Merkoçi, A. Micromotor enhanced microarray technology for protein detection. Small 10, 2542–2548 (2014).
Lin, M. et al. Programmable engineering of a biosensing interface with tetrahedral DNA nanostructures for ultrasensitive DNA detection. Angew. Chem. 127, 2179–2183 (2015).
Zhang, P. et al. Ultrasensitive detection of circulating exosomes with a 3D-nanopatterned microfluidic chip. Nat. Biomed. Eng. 3, 438–451 (2019).
Rivnay, J. et al. Organic electrochemical transistors. Nat. Rev. Mater. 3, 17086 (2018). This review describes the evolution of organic electrochemical transistors, including their fabrication, various configurations and their ability to amplify biochemical signals.
Rivnay, J. et al. Organic electrochemical transistors with maximum transconductance at zero gate bias. Adv. Mater. 25, 7010–7014 (2013).
Pappa, A. M. et al. Direct metabolite detection with an n-type accumulation mode organic electrochemical transistor. Sci. Adv. 4, eaat0911 (2018).
Liang, Y., Wu, C., Figueroa-Miranda, G., Offenhäusser, A. & Mayer, D. Amplification of aptamer sensor signals by four orders of magnitude via interdigitated organic electrochemical transistors. Biosens. Bioelectron. 144, 111668 (2019).
Ersman, P. A. et al. All-printed large-scale integrated circuits based on organic electrochemical transistors. Nat. Commun. 10, 5053 (2019).
Jarczewska, M., Rębiś, J., Górski, Ł. & Malinowska, E. Development of DNA aptamer-based sensor for electrochemical detection of C-reactive protein. Talanta 189, 45–54 (2018).
Reiber, T., Zavoiura, O., Dose, C. & Yushchenko, D. A. Fluorophore multimerization as an efficient approach towards bright protein labels. Eur. J. Org. Chem. 2021, 2817–2830 (2021).
Nakatsuka, N. et al. Aptamer-field-effect transistors overcome Debye length limitations for small-molecule sensing. Science 362, 319–324 (2018).
Kesler, V., Murmann, B. & Soh, H. T. Going beyond the Debye length: overcoming charge screening limitations in next-generation bioelectronic sensors. ACS Nano 14, 16194–16201 (2020).
Fu, K. et al. Accelerated electron transfer in nanostructured electrodes improves the sensitivity of electrochemical biosensors. Adv. Sci. 8, 2102495 (2021).
Lee, D. et al. Ionic contrast across a lipid membrane for Debye length extension: towards an ultimate bioelectronic transducer. Nat. Commun. 12, 3741 (2021).
Gao, W. et al. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature 529, 509–514 (2016).
Cui, F., Yue, Y., Zhang, Y., Zhang, Z. & Zhou, H. S. Advancing biosensors with machine learning. ACS Sens. 5, 3346–3364 (2020).
King, R. C. et al. Application of data fusion techniques and technologies for wearable health monitoring. Med. Eng. Phys. 42, 1–12 (2017).
Habib, C., Makhoul, A., Darazi, R. & Salim, C. Self-adaptive data collection and fusion for health monitoring based on body sensor networks. IEEE Trans. Ind. Inf. 12, 2342–2352 (2016).
Yu, X., Yang, Y.-P., Dikici, E., Deo, S. K. & Daunert, S. Beyond antibodies as binding partners: the role of antibody mimetics in bioanalysis. Annu. Rev. Anal. Chem. 10, 293–320 (2017).
Belbruno, J. J. Molecularly imprinted polymers. Chem. Rev. 119, 94–119 (2019).
Muyldermans, S. Nanobodies: natural single-domain antibodies. Annu. Rev. Biochem. 82, 775–797 (2013).
Silverman, J. et al. Multivalent avimer proteins evolved by exon shuffling of a family of human receptor domains. Nat. Biotechnol. 23, 1556–1561 (2005).
Tiede, C. et al. Affimer proteins are versatile and renewable affinity reagents. eLife 6, e24903 (2017).
Löfblom, J. et al. Affibody molecules: engineered proteins for therapeutic, diagnostic and biotechnological applications. FEBS Lett. 584, 2670–2680 (2010).
Bratkovič, T. Progress in phage display: evolution of the technique and its applications. Cell. Mol. Life Sci. 67, 749–767 (2010).
Bradbury, A. R. M., Sidhu, S., Dübel, S. & McCafferty, J. Beyond natural antibodies: the power of in vitro display technologies. Nat. Biotechnol. 29, 245–254 (2011).
Tuerk, C. & Gold, L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 249, 505–510 (1990).
Ellington, A. D. & Szostak, J. W. In vitro selection of RNA molecules that bind specific ligands. Nature 346, 818–822 (1990).
Qian, S., Chang, D., He, S. & Li, Y. Aptamers from random sequence space: accomplishments, gaps and future considerations. Anal. Chim. Acta 1196, 339511 (2022).
Yu, H., Alkhamis, O., Canoura, J., Liu, Y. & Xiao, Y. Advances and challenges in small-molecule DNA aptamer isolation, characterization, and sensor development. Angew. Chem. Int. Edn 60, 16800–16823 (2021).
Wang, B. et al. Wearable aptamer-field-effect transistor sensing system for noninvasive cortisol monitoring. Sci. Adv. 8, eabk0967 (2022). This article demonstrates a fully integrated body-based sensing platform for the continuous detection of cortisol using field-effect transistors and aptamer recognition elements.
Zhao, C. et al. Implantable aptamer-field-effect transistor neuroprobes for in vivo neurotransmitter monitoring. Sci. Adv. 7, eabj7422 (2021).
Frutiger, A. et al. Nonspecific binding — fundamental concepts and consequences for biosensing applications. Chem. Rev. 121, 8095–8160 (2021). This review provides a detailed analysis of non-specific binding, including its history, physiological causes and solutions to overcome its effects.
Gawande, B. N. et al. Selection of DNA aptamers with two modified bases. Proc. Natl Acad. Sci. USA 114, 2898–2903 (2017).
Yoshikawa, A. M. et al. Discovery of indole-modified aptamers for highly specific recognition of protein glycoforms. Nat. Commun. 12, 7106 (2021).
Wang, J. et al. Multiparameter particle display (MPPD): a quantitative screening method for the discovery of highly specific aptamers. Angew. Chem. Int. Edn Engl. 129, 762–765 (2017).
Zhou, J. & Rossi, J. Aptamers as targeted therapeutics: current potential and challenges. Nat. Rev. Drug. Discov. 16, 181–202 (2017).
Gerling, T., Kube, M., Kick, B. & Dietz, H. Sequence-programmable covalent bonding of designed DNA assemblies. Sci. Adv. 4, eaau1157 (2018).
Anastassacos, F. M., Zhao, Z., Zeng, Y. & Shih, W. M. Glutaraldehyde cross-linking of oligolysines coating DNA origami greatly reduces susceptibility to nuclease degradation. J. Am. Chem. Soc. 142, 3311–3315 (2020).
Biedermann, F. & Schneider, H.-J. Experimental binding energies in supramolecular complexes. Chem. Rev. 116, 5216–5300 (2016).
Bixler, G. D. & Bhushan, B. Biofouling: lessons from nature. Phil. Trans. R. Soc. A 370, 2381–2417 (2012).
Ostuni, E. et al. Self-assembled monolayers that resist the adsorption of proteins and the adhesion of bacterial and mammalian cells. Langmuir 17, 6336–6343 (2001).
Liu, S. & Guo, W. Anti-biofouling and healable materials: preparation, mechanisms, and biomedical applications. Adv. Funct. Mater. 28, 1800596 (2018).
Li, S. et al. Slippery liquid-infused microphase separation surface enables highly robust anti-fouling, anti-corrosion, anti-icing and anti-scaling coating on diverse substrates. Chem. Eng. J. 431, 133945 (2022).
Zhang, L. et al. Zwitterionic hydrogels implanted in mice resist the foreign-body reaction. Nat. Biotechnol. 31, 553–556 (2013).
Chan, D. et al. Combinatorial polyacrylamide hydrogels for preventing biofouling on implantable biosensors. Adv. Mater. 34, 2109764 (2022).
del Río, J. S., Henry, O. Y. F., Jolly, P. & Ingber, D. E. An antifouling coating that enables affinity-based electrochemical biosensing in complex biological fluids. Nat. Nanotechnol. 14, 1143–1149 (2019).
Timilsina, S. S. et al. Ultrarapid method for coating electrochemical sensors with antifouling conductive nanomaterials enables highly sensitive multiplexed detection in whole blood. Adv. Healthc. Mater. 11, 2102244 (2022).
Shiddiky, M. J. A., Vaidyanathan, R., Rauf, S., Tay, Z. & Trau, M. Molecular nanoshearing: an innovative approach to shear off molecules with AC-induced nanoscopic fluid flow. Sci. Rep. 4, 3716 (2014).
Xue, L. et al. Solid-state nanopore sensors. Nat. Rev. Mater. 5, 931–951 (2020).
Yang, B., Jiang, X., Fang, X. & Kong, J. Wearable chem-biosensing devices: from basic research to commercial market. Lab Chip 21, 4285–4310 (2021).
Fercher, C., Jones, M. L., Mahler, S. M. & Corrie, S. R. Recombinant antibody engineering enables reversible binding for continuous protein biosensing. ACS Sens. 6, 764–776 (2021).
Wilson, B. D. & Soh, H. T. Re-evaluating the conventional wisdom about binding assays. Trends Biochem. Sci. 45, 639–649 (2020).
Goode, J. A., Rushworth, J. V. H. & Millner, P. A. Biosensor regeneration: a review of common techniques and outcomes. Langmuir 31, 6267–6276 (2015).
Delport, F. et al. Real-time monitoring of DNA hybridization and melting processes using a fiber optic sensor. Nanotechnology 23, 065503 (2012).
Gilles, P. N., Wu, D. J., Foster, C. B., Dillon, P. J. & Chanock, S. J. Single nucleotide polymorphic discrimination by an electronic dot blot assay on semiconductor microchips. Nat. Biotechnol. 17, 365–370 (1999).
Clifford, A. et al. Strategies for biomolecular analysis and continuous physiological monitoring. J. Am. Chem. Soc. 143, 5281–5294 (2021).
Xiao, Y., Lubin, A. A., Heeger, A. J. & Plaxco, K. W. Label-free electronic detection of thrombin in blood serum by using an aptamer-based sensor. Angew. Chem. Int. Edn Engl. 44, 5456–5459 (2005).
Arroyo-Currás, N. et al. Real-time measurement of small molecules directly in awake, ambulatory animals. Proc. Natl Acad. Sci. USA 114, 645–650 (2017).
Dauphin-Ducharme, P., Ploense, K. L., Arroyo-Currás, N., Kippin, T. E. & Plaxco, K. W. Electrochemical aptamer-based sensors: a platform approach to high-frequency molecular monitoring in situ in the living body. Biomed. Eng. Technol. 2393, 479–492 (2022).
Ferguson, B. S. et al. Real-time, aptamer-based tracking of circulating therapeutic agents in living animals. Sci. Transl. Med. 5, 213ra165 (2013).
Idili, A., Gerson, J., Kippin, T. & Plaxco, K. W. Seconds-resolved, in situ measurements of plasma phenylalanine disposition kinetics in living rats. Anal. Chem. 93, 4023–4032 (2021).
Seo, J. W. et al. Real-time monitoring of drug pharmacokinetics within tumor tissue in live animals. Sci. Adv. 8, eabk2901 (2022). This article describes the real-time monitoring of doxorubicin in animal tumour tissue, demonstrating the effectiveness of reagentless sensing approaches in animal models.
Fan, C., Plaxco, K. W. & Heeger, A. J. Electrochemical interrogation of conformational changes as a reagentless method for the sequence-specific detection of DNA. Proc. Natl Acad. Sci. USA 100, 9134–9137 (2003).
Xiao, Y., Lubin, A. A., Baker, B. R., Plaxco, K. W. & Heeger, A. J. Single-step electronic detection of femtomolar DNA by target-induced strand displacement in an electrode-bound duplex. Proc. Natl Acad. Sci. USA 103, 16677–16680 (2006).
Li, C. et al. Design of DNA nanostructure-based interfacial probes for the electrochemical detection of nucleic acids directly in whole blood. Chem. Sci. 9, 979–984 (2018).
Yu, H. L. L., Maslova, A. & Hsing, I.-M. Rational design of electrochemical DNA biosensors for point-of-care applications. ChemElectroChem 4, 795–805 (2017).
Wang, M. et al. A reagentless triplex DNA junctions-based electrochemical DNA sensor using signal amplification strategy of CHA and tetraferrocene. Sens. Actuators B 358, 131496 (2022).
Ranallo, S., Porchetta, A. & Ricci, F. DNA-based scaffolds for sensing applications. Anal. Chem. 91, 44–59 (2019).
Parolo, C. et al. E-DNA scaffold sensors and the reagentless, single-step, measurement of HIV-diagnostic antibodies in human serum. Microsyst. Nanoeng. 6, 13 (2020).
Cash, K. J., Ricci, F. & Plaxco, K. W. An electrochemical sensor for the detection of protein–small molecule interactions directly in serum and other complex matrices. J. Am. Chem. Soc. 131, 6955–6957 (2009).
White, R. J. et al. Wash-free, electrochemical platform for the quantitative, multiplexed detection of specific antibodies. Anal. Chem. 84, 1098–1103 (2012).
Ogden, N. E., Kurnik, M., Parolo, C. & Plaxco, K. W. An electrochemical scaffold sensor for rapid syphilis diagnosis. Analyst 144, 5277–5283 (2019).
Ricci, F., Bonham, A. J., Mason, A. C., Reich, N. O. & Plaxco, K. W. Reagentless, electrochemical approach for the specific detection of double- and single-stranded DNA binding proteins. Anal. Chem. 81, 1608–1614 (2009).
Kurnik, M., Pang, E. Z. & Plaxco, K. W. An electrochemical biosensor architecture based on protein folding supports direct real-time measurements in whole blood. Angew. Chem. Int. Edn 59, 18442–18445 (2020).
Kang, D. et al. New architecture for reagentless, protein-based electrochemical biosensors. J. Am. Chem. Soc. 139, 12113–12116 (2017).
Das, J. et al. Reagentless biomolecular analysis using a molecular pendulum. Nat. Chem. 13, 428–434 (2021).
Yousefi, H. et al. Detection of SARS-CoV-2 viral particles using direct, reagent-free electrochemical sensing. J. Am. Chem. Soc. 143, 1722–1727 (2021).
Bertok, T. et al. Electrochemical impedance spectroscopy based biosensors: mechanistic principles, analytical examples and challenges towards commercialization for assays of protein cancer biomarkers. ChemElectroChem 6, 989–1003 (2019).
Flauzino, J. M. R. et al. Label-free and reagentless electrochemical genosensor based on graphene acid for meat adulteration detection. Biosens. Bioelectron. 195, 113628 (2022).
Cecchetto, J., Fernandes, F. C. B., Lopes, R. & Bueno, P. R. The capacitive sensing of NS1 Flavivirus biomarker. Biosens. Bioelectron. 87, 949–956 (2017).
Capaldo, P. et al. Circulating disease biomarker detection in complex matrices: real-time, in situ measurements of DNA/miRNA hybridization via electrochemical impedance spectroscopy. ACS Sens. 1, 1003–1010 (2016).
Luo, X., Xu, M., Freeman, C., James, T. & Davis, J. J. Ultrasensitive label free electrical detection of insulin in neat blood serum. Anal. Chem. 85, 4129–4134 (2013).
Kergoat, L., Piro, B., Berggren, M., Horowitz, G. & Pham, M.-C. Advances in organic transistor-based biosensors: from organic electrochemical transistors to electrolyte-gated organic field-effect transistors. Anal. Bioanal. Chem. 402, 1813–1826 (2012).
Hajian, R. et al. Detection of unamplified target genes via CRISPR–Cas9 immobilized on a graphene field-effect transistor. Nat. Biomed. Eng. 3, 427–437 (2019).
Wang, L. et al. Rapid and ultrasensitive electromechanical detection of ions, biomolecules and SARS-CoV-2 RNA in unamplified samples. Nat. Biomed. Eng. 6, 276–285 (2022).
Tai, T. Y. et al. Design and demonstration of tunable amplified sensitivity of AlGaN/GaN high electron mobility transistor (HEMT)-based biosensors in human serum. Anal. Chem. 91, 5953–5960 (2019).
Tyagi, S., Bratu, D. P. & Kramer, F. R. Multicolor molecular beacons for allele discrimination. Nat. Biotechnol. 16, 49–53 (1998).
Tuleuova, N. et al. Development of an aptamer beacon for detection of interferon-gamma. Anal. Chem. 82, 1851–1857 (2010).
Bai, Y., Shu, T., Su, L. & Zhang, X. Functional nucleic acid-based fluorescence polarization/anisotropy biosensors for detection of biomarkers. Anal. Bioanal. Chem. 412, 6655–6665 (2020).
Kruse, M. et al. Measuring influenza A virus and peptide interaction using electrically controllable DNA nanolevers. Adv. Mater. Technol. 7, 2101141 (2021).
Nguyen, P. Q. et al. Wearable materials with embedded synthetic biology sensors for biomolecule detection. Nat. Biotechnol. 39, 1366–1374 (2021).
Oh, S.-H. et al. Nanophotonic biosensors harnessing van der Waals materials. Nat. Commun. 12, 3824 (2021).
Aitekenov, S., Gaipov, A. & Bukasov, R. Review: Detection and quantification of proteins in human urine. Talanta 223, 121718 (2021).
Ploussard, G. & de La Taille, A. Urine biomarkers in prostate cancer. Nat. Rev. Urol. 7, 101–109 (2010).
Gray, M. et al. Implantable biosensors and their contribution to the future of precision medicine. Vet. J. 239, 21–29 (2018).
Soler, M. et al. Multiplexed nanoplasmonic biosensor for one-step simultaneous detection of Chlamydia trachomatis and Neisseria gonorrhoeae in urine. Biosens. Bioelectron. 94, 560–567 (2017).
Zhang, J. et al. A wearable self-powered biosensor system integrated with diaper for detecting the urine glucose of diabetic patients. Sens. Actuators B 341, 130046 (2021).
Lager, W. et al. Implantable electrocatalytic glucose sensor. Horm. Metab. Res. 26, 526–530 (1994).
Topkas, E., Keith, P., Dimeski, G., Cooper-White, J. & Punyadeera, C. Evaluation of saliva collection devices for the analysis of proteins. Clin. Chim. Acta 413, 1066–1070 (2012).
Zhang, C.-Z. et al. Saliva in the diagnosis of diseases. Int. J. Oral. Sci. 8, 133–137 (2016).
Bel’skaya, L. V., Sarf, E. A. & Kosenok, V. K. Age and gender characteristics of the biochemical composition of saliva: correlations with the composition of blood plasma. J. Oral Biol. Craniofac. Res. 10, 59–65 (2020).
Lin, C. et al. Toward the development of a glucose dehydrogenase-based saliva glucose sensor without the need for sample preparation. J. Diabetes Sci. Technol. 12, 83–89 (2018).
Arakawa, T. et al. Mouthguard biosensor with telemetry system for monitoring of saliva glucose: a novel cavitas sensor. Biosens. Bioelectron. 84, 106–111 (2016).
García-Carmona, L. et al. Pacifier biosensor: toward noninvasive saliva biomarker monitoring. Anal. Chem. 91, 13883–13891 (2019).
Kevadiya, B. D. et al. Diagnostics for SARS-CoV-2 infections. Nat. Mater. 20, 593–605 (2021).
Bariya, M., Nyein, H. Y. Y. & Javey, A. Wearable sweat sensors. Nat. Electron. 1, 160–171 (2018).
la Count, T. D., Jajack, A., Heikenfeld, J. & Kasting, G. B. Modeling glucose transport from systemic circulation to sweat. J. Pharm. Sci. 108, 364–371 (2019).
He, W. et al. Integrated textile sensor patch for real-time and multiplex sweat analysis. Sci. Adv. 5, eaax0649 (2019).
Pérez, D. & Orozco, J. Wearable electrochemical biosensors to measure biomarkers with complex blood-to-sweat partition such as proteins and hormones. Microchim. Acta 189, 127 (2022).
Cizza, G. et al. Elevated neuroimmune biomarkers in sweat patches and plasma of premenopausal women with major depressive disorder in remission: the P.O.W.E.R. study. Biol. Psychiat. 64, 907–911 (2008).
Qiao, L., Benzigar, M. R., Subramony, J. A., Lovell, N. H. & Liu, G. Advances in sweat wearables: sample extraction, real-time biosensing, and flexible platforms. ACS Appl. Mater. Interf. 12, 34337–34361 (2020).
Jagannath, B. et al. Novel approach to track the lifecycle of inflammation from chemokine expression to inflammatory proteins in sweat using electrochemical biosensor. Adv. Mater. Technol. 7, 2101356 (2022).
Jagannath, B. et al. Temporal profiling of cytokines in passively expressed sweat for detection of infection using wearable device. Bioeng. Transl. Med. 6, e10220 (2021).
Heikenfeld, J. et al. Accessing analytes in biofluids for peripheral biochemical monitoring. Nat. Biotechnol. 37, 407–419 (2019). This review discusses opportunities and challenges for biomolecular sensing in various biological fluids, with information on biological fluid generation, analyte partitioning and sampling technologies.
Kaya, T. et al. Wearable sweat sensors: background and current trends. Electroanalysis 31, 411–421 (2019).
You, J. et al. Tear fluid protein biomarkers. Adv. Clin. Chem. 62, 151–196 (2013).
Farandos, N. M., Yetisen, A. K., Monteiro, M. J., Lowe, C. R. & Yun, S. H. Contact lens sensors in ocular diagnostics. Adv. Healthc. Mater. 4, 792–810 (2015).
la Belle, J. T. et al. Self-monitoring of tear glucose: the development of a tear based glucose sensor as an alternative to self-monitoring of blood glucose. Chem. Commun. 52, 9197–9204 (2016).
Kim, J. et al. Wearable smart sensor systems integrated on soft contact lenses for wireless ocular diagnostics. Nat. Commun. 8, 14997 (2017).
Elsherif, M., Hassan, M. U., Yetisen, A. K. & Butt, H. Wearable contact lens biosensors for continuous glucose monitoring using smartphones. ACS Nano 12, 5452–5462 (2018).
Kim, J., Campbell, A. S., de Ávila, B. E. F. & Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 37, 389–406 (2019).
Sempionatto, J. R., Jeerapan, I., Krishnan, S. & Wang, J. Wearable chemical sensors: emerging systems for on-body analytical chemistry. Anal. Chem. 92, 378–396 (2020).
Stuchell, R. N., Feldman, J. J., Farris, R. L. & Mandel, I. D. The effect of collection technique on tear composition. Invest. Ophthalmol. Vis. Sci. 25, 374–377 (1984).
Kim, Y. & Prausnitz, M. R. Sensitive sensing of biomarkers in interstitial fluid. Nat. Biomed. Eng. 5, 3–5 (2021).
Vermeer, B. J., Reman, F. C. & van Gent, C. M. The determination of lipids and proteins in suction blister fluid. J. Investig. Dermatol. 73, 303–305 (1979).
Basu, A. et al. Time lag of glucose from intravascular to interstitial compartment in humans. Diabetes 62, 4083–4087 (2013).
Soni, A. et al. A practical approach to continuous glucose monitoring (rtCGM) and FreeStyle Libre systems (isCGM) in children and young people with type 1 diabetes. Diabetes Res. Clin. Pract. 184, 109196 (2022).
Samant, P. P. et al. Sampling interstitial fluid from human skin using a microneedle patch. Sci. Transl. Med. 12, eaaw0285 (2020).
Samant, P. P. & Prausnitz, M. R. Mechanisms of sampling interstitial fluid from skin using a microneedle patch. Proc. Natl Acad. Sci. USA 115, 4583–4588 (2018).
Friedel, M. et al. Opportunities and challenges in the diagnostic utility of dermal interstitial fluid. Nat. Biomed. Eng. https://doi.org/10.1038/s41551-022-00998-9 (2023). This Perspective provides a detailed analysis of the opportunities and challenges related to interstitial-fluid-based sensing and provides a roadmap for future interstitial fluid sensor development.
Vasilescu, A., Hrinczenko, B., Swain, G. M. & Peteu, S. F. Exhaled breath biomarker sensing. Biosens. Bioelectron. 182, 113193 (2021).
Chen, H. et al. Automated in vivo nanosensing of breath-borne protein biomarkers. Nano Lett. 18, 4716–4726 (2018).
Ates, H. C. & Dincer, C. Wearable breath analysis. Nat. Rev. Bioeng. 1, 80–82 (2023).
McBurney, M. I. et al. Establishing what constitutes a healthy human gut microbiome: state of the science, regulatory considerations, and future directions. J. Nutr. 149, 1882–1895 (2019).
Melton, S. D., Genta, R. M. & Souza, R. F. Biomarkers and molecular diagnosis of gastrointestinal and pancreatic neoplasms. Nat. Rev. Gastroenterol. Hepatol. 7, 620–628 (2010).
Salama, R., Arshavsky-Graham, S., Sella-Tavor, O., Massad-Ivanir, N. & Segal, E. Design considerations of aptasensors for continuous monitoring of biomarkers in digestive tract fluids. Talanta 239, 123124 (2022).
Ruiz-Valdepeñas Montiel, V. et al. Direct electrochemical biosensing in gastrointestinal fluids. Anal. Bioanal. Chem. 411, 4597–4604 (2019).
Mimee, M. et al. An ingestible bacterial-electronic system to monitor gastrointestinal health. Science 360, 915–918 (2018).
Beardslee, L. A. et al. Ingestible sensors and sensing systems for minimally invasive diagnosis and monitoring: the next frontier in minimally invasive screening. ACS Sens. 5, 891–910 (2020).
Yang, Y. & Gao, W. Wearable and flexible electronics for continuous molecular monitoring. Chem. Soc. Rev. 48, 1465–1491 (2019).
Wang, L., Xu, T. & Zhang, X. Multifunctional conductive hydrogel-based flexible wearable sensors. TrAC Trends Anal. Chem. 134, 116130 (2021).
Someya, T., Bao, Z. & Malliaras, G. G. The rise of plastic bioelectronics. Nature 540, 379–385 (2016).
Park, Y.-G. et al. Liquid metal-based soft electronics for wearable healthcare. Adv. Healthc. Mater. 10, 2002280 (2021).
Libanori, A., Chen, G., Zhao, X., Zhou, Y. & Chen, J. Smart textiles for personalized healthcare. Nat. Electron. 5, 142–156 (2022).
Wang, L. et al. Functionalized helical fibre bundles of carbon nanotubes as electrochemical sensors for long-term in vivo monitoring of multiple disease biomarkers. Nat. Biomed. Eng. 4, 159–171 (2020).
Shah, S. R., Tatara, A. M., D’Souza, R. N., Mikos, A. G. & Kasper, F. K. Evolving strategies for preventing biofilm on implantable materials. Mater. Today 16, 177–182 (2013).
Kim, J. et al. Edible electrochemistry: food materials based electrochemical sensors. Adv. Healthc. Mater. 6, 1700770 (2017).
Yin, L. et al. A passive perspiration biofuel cell: high energy return on investment. Joule 5, 1888–1904 (2021).
Hinchet, R. et al. Transcutaneous ultrasound energy harvesting using capacitive triboelectric technology. Science 365, 491–494 (2019).
Ali, F., Raza, W., Li, X., Gul, H. & Kim, K.-H. Piezoelectric energy harvesters for biomedical applications. Nano Energy 57, 879–902 (2019).
Jeong, Y. R., Lee, G., Park, H. & Ha, J. S. Stretchable, skin-attachable electronics with integrated energy storage devices for biosignal monitoring. Acc. Chem. Res. 52, 91–99 (2019).
Hajiaghajani, A. et al. Textile-integrated metamaterials for near-field multibody area networks. Nat. Electron. 4, 808–817 (2021).
Chung, J., Sepunaru, L. & Plaxco, K. W. On the disinfection of electrochemical aptamer-based sensors. ECS Sens. Plus 1, 011604 (2022).
Zhang, Y., Bindra, D. S., Barrau, M.-B. & Wilson, G. S. Application of cell culture toxicity tests to the development of implantable biosensors. Biosens. Bioelectron. 6, 653–661 (1991).
Avula, M. N., Rao, A. N., McGill, L. D., Grainger, D. W. & Solzbacher, F. Foreign body response to subcutaneous biomaterial implants in a mast cell-deficient Kit(w-Sh) murine model. Acta Biomater. 10, 1856–1863 (2014).
Fang, Y.-M. & Chang, C.-C. Users’ psychological perception and perceived readability of wearable devices for elderly people. Behav. Inf. Technol. 35, 225–232 (2016).
Pycroft, L. & Aziz, T. Z. Security of implantable medical devices with wireless connections: the dangers of cyber-attacks. Expert Rev. Med. Devices 15, 403–406 (2018).
Sadowski, J., Viljoen, S. & Whittaker, M. Everyone should decide how their digital data are used — not just tech companies. Nature 595, 169–171 (2021).
Teymourian, H. et al. Wearable electrochemical sensors for the monitoring and screening of drugs. ACS Sens. 5, 2679–2700 (2020).
Goud, K. Y. et al. Wearable electrochemical microneedle sensor for continuous monitoring of Levodopa: toward Parkinson management. ACS Sens. 4, 2196–2204 (2019).
Sempionatto, J. R., Montiel, V. R.-V., Vargas, E., Teymourian, H. & Wang, J. Wearable and mobile sensors for personalized nutrition. ACS Sens. 6, 1745–1760 (2021).
Thabit, H. & Hovorka, R. Coming of age: the artificial pancreas for type 1 diabetes. Diabetologia 59, 1795–1805 (2016).
Biosensors market size by type (wearable, non-wearable), by technology (electrochemical, optical, thermal, piezoelectric), by medical application (blood glucose testing, cholesterol testing, blood gas analysis, pregnancy testing, drug discovery. Global Market Insights https://www.gminsights.com/industry-analysis/biosensors-market (2022).
Acknowledgements
The authors thank A. Jiao for her illustrative contributions to the manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the writing of the manuscript. C.D.F. and S.O.K. edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
S.O.K. and H.Y. are cofounders and equity holders in Arma Biosciences, which is commercializing new sensing technologies.
Peer review
Peer review information
Nature Reviews Bioengineering thanks Chunhai Fan and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Flynn, C.D., Chang, D., Mahmud, A. et al. Biomolecular sensors for advanced physiological monitoring. Nat Rev Bioeng 1, 560–575 (2023). https://doi.org/10.1038/s44222-023-00067-z
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44222-023-00067-z
This article is cited by
-
Microsystem technologies for accelerating the discovery and translation of immunotherapies
Nature Reviews Drug Discovery (2026)
-
NFC/RFID-enabled wearables and implants for biomedical applications
Microsystems & Nanoengineering (2025)
-
Integrating bioelectronics with cell-based synthetic biology
Nature Reviews Bioengineering (2025)
-
Barriers to translating continuous monitoring technologies for preventative medicine
Nature Biomedical Engineering (2025)
-
Wearable biomolecular sensing nanotechnologies in chronic disease management
Nature Nanotechnology (2025)