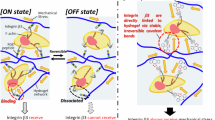
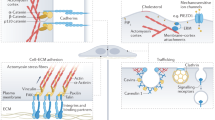
Abstract
Cell engineering has the aim of producing cells with controlled phenotype for medical use, for example, for cell therapy, cell transplantation or drug discovery. However, chemical induction of cell phenotypes, in particular, the use of inductive media and growth factors, often lacks specificity, might be unsuitable for clinical use and remains costly and difficult to scale up. Alternatively, mechanotransductive stimulation can be applied to engineer cells with specific phenotypes. In this Review, we discuss vibration as a mechanotransductive cell-engineering tool for both in vitro phenotypic control and in vivo regenerative therapy. We examine how vibration devices can be designed to provide specific frequencies and amplitudes to which cells respond through either adhesion-induced or channel-induced mechanotransduction pathways. We further highlight key applications of vibrational stimulation for bone regeneration as well as whole-body vibration as regenerative therapy, identifying important mechanisms of action and gaps in translational pipelines.
Key points
-
Cells can respond to their mechanical environment through phenotypic and functional changes.
-
Vibration is being explored for cell engineering to modify cellular growth, motility and differentiation.
-
Various vibration parameters (frequency, amplitude and duration) are being explored for cell engineering, but their relation to mechanotransductive signalling and phenotypic changes are yet to be fully understood.
-
Vibrational stimulation might be clinically applied for cell therapy or regenerative medicine, but scalibility and optimization of parameters remain challenging.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Jaklenec, A., Stamp, A., Deweerd, E., Sherwin, A. & Langer, R. Progress in the tissue engineering and stem cell industry “are we there yet?”. Tissue Eng. B 18, 155–166 (2012).
McPherson, J. M. & Tubo, R. Autologous chondrocyte transplantation (Carticel®): lessons learned and future challenges. In Vitro Cell. Dev. Biol. 40, 6A (2004).
Starzl, T. E. History of clinical transplantation. World J. Surg. 24, 759–782 (2000).
Childs, P. G., Reid, S., Salmeron-Sanchez, M. & Dalby, M. J. Hurdles to uptake of mesenchymal stem cells and their progenitors in therapeutic products. Biochem. J. 477, 3349–3366 (2020).
Yin, J. Q., Zhu, J. & Ankrum, J. A. Manufacturing of primed mesenchymal stromal cells for therapy. Nat. Biomed. Eng. 3, 90–104 (2019).
Martins, J. P. et al. Towards an advanced therapy medicinal product based on mesenchymal stromal cells isolated from the umbilical cord tissue: quality and safety data. Stem Cell Res. Ther. 5, 9 (2014).
Rozwadowska, N. et al. Optimization of human myoblasts culture under different media conditions for application in the in vitro studies. Am. J. Stem Cell 11, 1–11 (2022).
Sonnet, W. & Aznar‐López, C. Treatment of delayed-union fractures of long bones with minimally invasive administration of allogeneic bone-forming cells differentiated from mesenchymal stem cells: a pilot clinical trial. Bone Innov. 1, 3–7 (2019).
Czapla, J. et al. The effect of culture media on large-scale expansion and characteristic of adipose tissue-derived mesenchymal stromal cells. Stem Cell Res. Ther. 10, 235 (2019).
Gualdi, T. et al. In vitro osteodifferentiation of intact human amniotic membrane is not beneficial in the context of bone repair. Cell Tissue Bank. 20, 435–446 (2019).
Wang, N., Tytell, J. D. & Ingber, D. E. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat. Rev. Mol. Cell Biol. 10, 75–82 (2009).
Vining, K. H. & Mooney, D. J. Mechanical forces direct stem cell behaviour in development and regeneration. Nat. Rev. Mol. Cell Biol. 18, 728–742 (2017).
Wang, N. Review of cellular mechanotransduction. J. Phys. D 50, 233002 (2017).
Brusatin, G., Panciera, T., Gandin, A., Citron, A. & Piccolo, S. Biomaterials and engineered microenvironments to control YAP/TAZ-dependent cell behaviour. Nat. Mater. 17, 1063–1075 (2018).
Melo-Fonseca, F. et al. Reengineering bone–implant interfaces for improved mechanotransduction and clinical outcomes. Stem Cell Rev. Rep. 16, 1121–1138 (2020).
Discher, D. E., Janmey, P. & Wang, Y.-L. Tissue cells feel and respond to the stiffness of their substrate. Science 310, 1139–1143 (2005).
Cantini, M., Donnelly, H., Dalby, M. J. & Salmeron‐Sanchez, M. The plot thickens: the emerging role of matrix viscosity in cell mechanotransduction. Adv. Healthc. Mater. 9, 1901259 (2020).
Luciano, M. et al. Cell monolayers sense curvature by exploiting active mechanics and nuclear mechanoadaptation. Nat. Phys. 17, 1382–1390 (2021).
Roca-Cusachs, P., Conte, V. & Trepat, X. Quantifying forces in cell biology. Nat. Cell Biol. 19, 742–751 (2017).
Klumpers, D. D., Zhao, X., Mooney, D. J. & Smit, T. H. Cell mediated contraction in 3D cell-matrix constructs leads to spatially regulated osteogenic differentiation. Integr. Biol. 5, 1174–1183 (2013).
Hodgkinson, T. et al. The use of nanovibration to discover specific and potent bioactive metabolites that stimulate osteogenic differentiation in mesenchymal stem cells. Sci. Adv. 7, eabb7921 (2021).
Mirmalek-Sani, S. H. et al. Characterization and multipotentiality of human fetal femur-derived cells: implications for skeletal tissue regeneration. Stem Cell 24, 1042–1053 (2006).
Wu, J. et al. Joint construction of micro-vibration stimulation and BCP scaffolds for enhanced bioactivity and self-adaptability tissue engineered bone grafts. J. Mater. Chem. B 8, 4278–4288 (2020).
ElDeeb, A. M. & Abdel-Aziem, A. A. Effect of whole-body vibration exercise on power profile and bone mineral density in postmenopausal women with osteoporosis: a randomized controlled trial. J. Manip. Physiol. Ther. 43, 384–393 (2020).
Cooper, N. P., Vavakou, A. & van der Heijden, M. Vibration hotspots reveal longitudinal funneling of sound-evoked motion in the mammalian cochlea. Nat. Commun. 9, 3054 (2018).
Brown, G. N., Sattler, R. L. & Guo, X. E. Experimental studies of bone mechanoadaptation: bridging in vitro and in vivo studies with multiscale systems. Interface Focus. 6, 20150071 (2016).
Malone, A. M. et al. Primary cilia mediate mechanosensing in bone cells by a calcium-independent mechanism. Proc. Natl Acad. Sci. USA 104, 13325–13330 (2007).
Wang, J., Sun, Y.-X. & Li, J. The role of mechanosensor Piezo1 in bone homeostasis and mechanobiology. Dev. Biol. 493, 80–88 (2022).
Vanmunster, M. et al. Mechanosensors control skeletal muscle mass, molecular clocks, and metabolism. Cell. Mol. Life Sci. 79, 321 (2022).
Jaalouk, D. E. & Lammerding, J. Mechanotransduction gone awry. Nat. Rev. Mol. Cell Biol. 10, 63–73 (2009).
Jiao, Y. et al. The crescendo pulse frequency of shear stress stimulates the endothelialization of bone marrow mesenchymal stem cells on the luminal surface of decellularized scaffold in the bioreactor. Bioengineered 13, 7925–7938 (2022).
Tan, Y. et al. Low-intensity pulsed ultrasound stimulates proliferation of stem/progenitor cells: what we need to know to translate basic science research into clinical applications. Asian J. Androl. 23, 602 (2021).
Markides, H. et al. Translation of remote control regenerative technologies for bone repair. npj Regen. Med. 3, 9 (2018).
Corrigan, M. A. et al. TRPV4-mediates oscillatory fluid shear mechanotransduction in mesenchymal stem cells in part via the primary cilium. Sci. Rep. 8, 3824 (2018).
Hahn, C. & Schwartz, M. A. Mechanotransduction in vascular physiology and atherogenesis. Nat. Rev. Mol. Cell Biol. 10, 53–62 (2009).
Dupont, S. et al. Role of YAP/TAZ in mechanotransduction. Nature 474, 179–183 (2011).
Beijer, N. R. et al. Dynamic adaptation of mesenchymal stem cell physiology upon exposure to surface micropatterns. Sci. Rep. 9, 9099 (2019).
Pemberton, G. D. et al. Nanoscale stimulation of osteoblastogenesis from mesenchymal stem cells: nanotopography and nanokicking. Nanomedicine 10, 547–560 (2015).
Walther, B. K. et al. Mechanotransduction-on-chip: vessel-chip model of endothelial YAP mechanobiology reveals matrix stiffness impedes shear response. Lab Chip 21, 1738–1751 (2021).
Yarishkin, O. et al. Mechanotransduction and dynamic outflow regulation in trabecular meshwork requires Piezo1 channels. Preprint at bioRxiv https://doi.org/10.1101/2020.06.30.180653 (2020).
Melica, M. E. et al. Substrate stiffness modulates renal progenitor cell properties via a ROCK-mediated mechanotransduction mechanism. Cells 8, 1561 (2019).
Brown, T. D. Techniques for mechanical stimulation of cells in vitro: a review. J. Biomech. 33, 3–14 (2000).
Stavenschi, E., Labour, M.-N. & Hoey, D. A. Oscillatory fluid flow induces the osteogenic lineage commitment of mesenchymal stem cells: the effect of shear stress magnitude, frequency, and duration. J. Biomech. 55, 99–106 (2017).
Tsimbouri, P. M. et al. Stimulation of 3D osteogenesis by mesenchymal stem cells using a nanovibrational bioreactor. Nat. Biomed. Eng. 1, 758–770 (2017).
Song, Z., Banks, R. W. & Bewick, G. S. Modelling the mechanoreceptor’s dynamic behaviour. J. Anat. 227, 243–254 (2015).
Sawada, Y., Murase, M. & Sokabe, M. The gating mechanism of the bacterial mechanosensitive channel MscL revealed by molecular dynamics simulations: from tension sensing to channel opening. Channels 6, 317–331 (2012).
Appel, H. M. & Cocroft, R. B. Plants respond to leaf vibrations caused by insect herbivore chewing. Oecologia 175, 1257–1266 (2014).
von Muggenthaler, E. The felid purr: a healing mechanism? J. Acoust. Soc. Am. 110, 2666–2666 (2001).
Nikukar, H. et al. Osteogenesis of mesenchymal stem cells by nanoscale mechanotransduction. ACS Nano 7, 2758–2767 (2013).
Yang, C., Tibbitt, M. W., Basta, L. & Anseth, K. S. Mechanical memory and dosing influence stem cell fate. Nat. Mater. 13, 645–652 (2014).
Thompson, M., Woods, K., Newberg, J., Oxford, J. T. & Uzer, G. Low-intensity vibration restores nuclear YAP levels and acute YAP nuclear shuttling in mesenchymal stem cells subjected to simulated microgravity. npj Microgravity 6, 35 (2020).
Kanchanawong, P. et al. Nanoscale architecture of integrin-based cell adhesions. Nature 468, 580–584 (2010).
Kilian, K. A., Bugarija, B., Lahn, B. T. & Mrksich, M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proc. Natl Acad. Sci. USA 107, 4872–4877 (2010).
Lee, W. et al. The osteogenic differentiation of human dental pulp stem cells through G0/G1 arrest and the p-ERK/Runx-2 pathway by sonic vibration. Int. J. Mol. Sci. 22, 10167 (2021).
Zhou, Y. et al. Osteogenic differentiation of bone marrow-derived mesenchymal stromal cells on bone-derived scaffolds: effect of microvibration and role of ERK1/2 activation. Eur. Cell Mater. 22, 12–25 (2011).
Lu, Y. et al. Vibration loading promotes osteogenic differentiation of bone marrow-derived mesenchymal stem cells via p38 MAPK signaling pathway. J. Biomech. 71, 67–75 (2018).
Wu, R. W. et al. Piezoelectric microvibration mitigates estrogen loss-induced osteoporosis and promotes piezo1, microRNA-29a, and Wnt3a signaling in osteoblasts. Int. J. Mol. Sci. 22, 9476 (2021).
Bas, G. et al. Low intensity vibrations augment mesenchymal stem cell proliferation and differentiation capacity during in vitro expansion. Sci. Rep. 10, 9369 (2020).
Halonen, H. T., Ihalainen, T. O., Hyväri, L., Miettinen, S. & Hyttinen, J. A. Cell adhesion and culture medium dependent changes in the high frequency mechanical vibration induced proliferation, osteogenesis, and intracellular organization of human adipose stem cells. J. Mech. Behav. Biomed. Mater. 101, 103419 (2020).
Hou, W., Zhang, D., Feng, X. & Zhou, Y. Low magnitude high frequency vibration promotes chondrogenic differentiation of bone marrow stem cells with involvement of β-catenin signaling pathway. Arch. Oral. Biol. 118, 104860 (2020).
Safavi, A. S. et al. Efficacy of mechanical vibration in regulating mesenchymal stem cells gene expression. In Vitro Cell. Dev. Biol. Anim. 55, 387–394 (2019).
Anggayasti, W. L., Imashiro, C., Kuribara, T., Totani, K. & Takemura, K. Low‐frequency mechanical vibration induces apoptosis of A431 epidermoid carcinoma cells. Eng. Life Sci. 20, 232–238 (2020).
Zhao, Q., Lu, Y., Gan, X. & Yu, H. Low magnitude high frequency vibration promotes adipogenic differentiation of bone marrow stem cells via P38 MAPK signal. PLoS ONE 12, e0172954 (2017).
Pongkitwitoon, S., Uzer, G., Rubin, J. & Judex, S. Cytoskeletal configuration modulates mechanically induced changes in mesenchymal stem cell osteogenesis, morphology, and stiffness. Sci. Rep. 6, 34791 (2016).
Tapia-Rojo, R., Alonso-Caballero, Á. & Fernández, J. M. Talin folding as the tuning fork of cellular mechanotransduction. Proc. Natl Acad. Sci. USA 117, 21346–21353 (2020).
Griffin, X. L., Parsons, N., Costa, M. L. & Metcalfe, D. Ultrasound and shockwave therapy for acute fractures in adults. Cochrane Datab. Syst. Rev. 3, CD008579 (2014).
Lam, T. et al. Effect of whole body vibration (WBV) therapy on bone density and bone quality in osteopenic girls with adolescent idiopathic scoliosis: a randomized, controlled trial. Osteopor. Int. 24, 1623–1636 (2013).
Ambattu, L. A. & Yeo, L. Y. Sonomechanobiology: vibrational stimulation of cells and its therapeutic implications. Biophys. Rev. 4, 021301 (2023).
Ling, L. et al. Low‐intensity pulsed ultrasound activates ERK1/2 and PI3K‐Akt signalling pathways and promotes the proliferation of human amnion‐derived mesenchymal stem cells. Cell Prolif. 50, e12383 (2017).
Wang, Y. et al. Low-intensity pulsed ultrasound promotes periodontal ligament stem cell migration through TWIST1-mediated SDF-1 expression. Int. J. Mol. Med. 42, 322–330 (2018); corrigendum 49, 38 (2022).
Wang, Y. et al. Study of bilineage differentiation of human-bone-marrow-derived mesenchymal stem cells in oxidized sodium alginate/N-succinyl chitosan hydrogels and synergistic effects of RGD modification and low-intensity pulsed ultrasound. Acta Biomater. 10, 2518–2528 (2014).
Snehota, M., Vachutka, J., Ter Haar, G., Dolezal, L. & Kolarova, H. Therapeutic ultrasound experiments in vitro: review of factors influencing outcomes and reproducibility. Ultrasonics 107, 106167 (2020).
Gupta, D. et al. Traditional multiwell plates and petri dishes limit the evaluation of the effects of ultrasound on cells in vitro. Ultrasound Med. Biol. 48, 1745–1761 (2022).
Campsie, P. et al. Design, construction and characterisation of a novel nanovibrational bioreactor and cultureware for osteogenesis. Sci. Rep. 9, 12944 (2019).
Prè, D. et al. High-frequency vibration treatment of human bone marrow stromal cells increases differentiation toward bone tissue. Bone Marrow Res. 2013, 803450 (2013).
Uzer, G. et al. Separating fluid shear stress from acceleration during vibrations in vitro: identification of mechanical signals modulating the cellular response. Cell. Mol. Bioeng. 5, 266–276 (2012).
Ito, Y. et al. Effects of vibration on differentiation of cultured PC12 cells. Biotechnol. Bioeng. 108, 592–599 (2011).
Choi, S. & Kuchenbecker, K. J. Vibrotactile display: perception, technology, and applications. Proc. IEEE 101, 2093–2104 (2012).
Dong, S. Review on piezoelectric, ultrasonic, and magnetoelectric actuators. J. Adv. Dielectr. 2, 1230001 (2012).
Hensel, K., Mienkina, M. P. & Schmitz, G. Analysis of ultrasound fields in cell culture wells for in vitro ultrasound therapy experiments. Ultrasound Med. Biol. 37, 2105–2115 (2011).
Patel, U. S. et al. Ultrasound field characterization and bioeffects in multiwell culture plates. J. Ther. Ultrasound 3, 8 (2015).
LaGier, A. J., Elbe, A., Thamke, A. & Anderson, P. Vibration, a treatment for migraine, linked to calpain driven changes in actin cytoskeleton. PLoS ONE 17, e0262058 (2022).
Tirkkonen, L. et al. The effects of vibration loading on adipose stem cell number, viability and differentiation towards bone-forming cells. J. R. Soc. Interf. 8, 1736–1747 (2011).
Bacabac, R. G. et al. Bone cell responses to high-frequency vibration stress: does the nucleus oscillate within the cytoplasm? FASEB J. 20, 858–864 (2006).
Prè, D., Ceccarelli, G., Benedetti, L., Magenes, G. & De Angelis, M. G. Effects of low-amplitude, high-frequency vibrations on proliferation and differentiation of SAOS-2 human osteogenic cell line. Tissue Eng. C 15, 669–679 (2009).
Prè, D. et al. The differentiation of human adipose-derived stem cells (hASCs) into osteoblasts is promoted by low amplitude, high frequency vibration treatment. Bone 49, 295–303 (2011).
Nikukar, H. et al. Production of nanoscale vibration for stimulation of human mesenchymal stem cells. J. Biomed. Nanotechnol. 12, 1478–1488 (2016).
Childs, P. G. et al. Use of nanoscale mechanical stimulation for control and manipulation of cell behaviour. Acta Biomater. 34, 159–168 (2016).
Orapiriyakul, W. et al. Nanovibrational stimulation of mesenchymal stem cells induces therapeutic reactive oxygen species and inflammation for three-dimensional bone tissue engineering. ACS Nano 14, 10027–10044 (2020).
Abbott, B. P. et al. Observation of gravitational waves from a binary black hole merger. Phys. Rev. Lett. 116, 061102 (2016).
Gao, H. et al. Low-level mechanical vibration enhances osteoblastogenesis via a canonical Wnt signaling-associated mechanism. Mol. Med. Rep. 16, 317–324 (2017).
Enomoto, U., Imashiro, C. & Takemura, K. Collective cell migration of fibroblasts is affected by horizontal vibration of the cell culture dish. Eng. Life Sci. 20, 402–411 (2020).
Mojena-Medina, D. et al. Design, implementation, and validation of a piezoelectric device to study the effects of dynamic mechanical stimulation on cell proliferation, migration and morphology. Sensors 20, 2155 (2020).
Ota, T., Chiba, M. & Hayashi, H. Vibrational stimulation induces osteoblast differentiation and the upregulation of osteogenic gene expression in vitro. Cytotechnology 68, 2287–2299 (2016).
Sancilio, S. et al. Effects of focused vibrations on human satellite cells. Int. J. Mol. Sci. 23, 6026 (2022).
Takeuchi, R. et al. Effects of vibration and hyaluronic acid on activation of three-dimensional cultured chondrocytes. Arthritis Rheum. 54, 1897–1905 (2006).
Grosman-Dziewiszek, P. et al. Influence of 40 Hz and 100 Hz vibration on SH-SY5Y cells growth and differentiation—a preliminary study. Molecules 27, 3337 (2022).
Lin, C. Y. et al. Yoda1 enhanced low-magnitude high-frequency vibration on osteocytes in regulation of MDA-MB-231 breast cancer cell migration. Cancers 14, 3395 (2022).
Uzer, G. et al. Cell mechanosensitivity to extremely low-magnitude signals is enabled by a LINCed nucleus. Stem Cell 33, 2063–2076 (2015).
Lau, E. et al. Effect of low-magnitude, high-frequency vibration on osteogenic differentiation of rat mesenchymal stromal cells. J. Orthop. Res. 29, 1075–1080 (2011).
Uzer, G., Pongkitwitoon, S., Ete Chan, M. & Judex, S. Vibration induced osteogenic commitment of mesenchymal stem cells is enhanced by cytoskeletal remodeling but not fluid shear. J. Biomech. 46, 2296–2302 (2013).
Lorusso, D., Nikolov, H. N., Holdsworth, D. W. & Dixon, S. J. Vibration of osteoblastic cells using a novel motion-control platform does not acutely alter cytosolic calcium, but desensitizes subsequent responses to extracellular ATP. J. Cell. Physiol. 235, 5096–5110 (2020).
Coughlin, T. R. & Niebur, G. L. Fluid shear stress in trabecular bone marrow due to low-magnitude high-frequency vibration. J. Biomech. 45, 2222–2229 (2012).
Ambattu, L. A., Gelmi, A. & Yeo, L. Y. Short-duration high frequency megahertz-order nanomechanostimulation drives early and persistent osteogenic differentiation in mesenchymal stem cells. Small 18, 2106823 (2022).
Kennedy, J. W. et al. Nanovibrational stimulation inhibits osteoclastogenesis and enhances osteogenesis in co-cultures. Sci. Rep. 11, 22741 (2021).
Tong, Z., Duncan, R. & Jia, X. Modulating the behaviors of mesenchymal stem cells via the combination of high-frequency vibratory stimulations and fibrous scaffolds. Tissue Eng. A 19, 1862–1878 (2013).
Stein, G. S. & Lian, J. B. Molecular mechanisms mediating proliferation/differentiation interrelationships during progressive development of the osteoblast phenotype. Endocr. Rev. 14, 424–442 (1993).
Yang, J. et al. Nanotopographical induction of osteogenesis through adhesion, bone morphogenic protein cosignaling, and regulation of microRNAs. ACS Nano 8, 9941–9953 (2014).
Li, Y. H. et al. Primary cilia respond to intermittent low-magnitude, high-frequency vibration and mediate vibration-induced effects in osteoblasts. Am. J. Physiol. Cell Physiol. 318, C73–c82 (2020).
Judex, S. & Pongkitwitoon, S. Differential efficacy of 2 vibrating orthodontic devices to alter the cellular response in osteoblasts, fibroblasts, and osteoclasts. Dose Response 16, 1559325818792112 (2018).
Marędziak, M., Lewandowski, D., Tomaszewski, K. A., Kubiak, K. & Marycz, K. The effect of low-magnitude low-frequency vibrations (LMLF) on osteogenic differentiation potential of human adipose derived mesenchymal stem cells. Cell Mol. Bioeng. 10, 549–562 (2017).
Rosenberg, N., Levy, M. & Francis, M. Experimental model for stimulation of cultured human osteoblast-like cells by high frequency vibration. Cytotechnology 39, 125–130 (2002).
Marycz, K. et al. Low-frequency, low-magnitude vibrations (LFLM) enhances chondrogenic differentiation potential of human adipose derived mesenchymal stromal stem cells (hASCs). PeerJ 4, e1637 (2016).
Chen, X., He, F., Zhong, D.-Y. & Luo, Z.-P. Acoustic-frequency vibratory stimulation regulates the balance between osteogenesis and adipogenesis of human bone marrow-derived mesenchymal stem cells. Biomed. Res. Int. 2015, 540731 (2015).
Baskan, O., Mese, G. & Ozcivici, E. Low-intensity vibrations normalize adipogenesis-induced morphological and molecular changes of adult mesenchymal stem cells. Proc. Inst. Mech. Eng. H 231, 160–168 (2017).
Baskan, O., Sarigil, O., Mese, G. & Ozcivici, E. Frequency-specific sensitivity of 3T3-L1 preadipocytes to low-intensity vibratory stimulus during adipogenesis. In Vitro Cell. Dev. Biol. Anim. 58, 452–461 (2022).
Cashion, A. T. et al. Programmable mechanobioreactor for exploration of the effects of periodic vibratory stimulus on mesenchymal stem cell differentiation. Biores. Open Access 3, 19–28 (2014).
Zhang, C. et al. Influence of different intensities of vibration on proliferation and differentiation of human periodontal ligament stem cells. Arch. Med. Sci. 11, 638–646 (2015).
Lee, J., Abdeen, A. A., Tang, X., Saif, T. A. & Kilian, K. A. Geometric guidance of integrin mediated traction stress during stem cell differentiation. Biomaterials 69, 174–183 (2015).
Kilian, K. A. & Mrksich, M. Directing stem cell fate by controlling the affinity and density of ligand-receptor interactions at the biomaterials interface. Angew. Chem. Int. Edn 51, 4891–4895 (2012).
McBeath, R., Pirone, D. M., Nelson, C. M., Bhadriraju, K. & Chen, C. S. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell 6, 483–495 (2004).
Engler, A. J., Sen, S., Sweeney, H. L. & Discher, D. E. Matrix elasticity directs stem cell lineage specification. Cell 126, 677–689 (2006).
Ross, E. A. et al. Nanotopography reveals metabolites that maintain the immunomodulatory phenotype of mesenchymal stromal cells. Nat. Commun. 14, 753 (2023).
Tsimbouri, P. M. et al. Using nanotopography and metabolomics to identify biochemical effectors of multipotency. ACS Nano 6, 10239–10249 (2012).
Knight, C. G. et al. The collagen-binding A-domains of integrins α1β1 and α2β1 recognize the same specific amino acid sequence, GFOGER, in native (triple-helical) collagens. J. Biol. Chem. 275, 35–40 (2000).
Dalby, M. J., Garcia, A. J. & Salmeron-Sanchez, M. Receptor control in mesenchymal stem cell engineering. Nat. Rev. Mater. 3, 17091 (2018).
Dalby, M. J., Gadegaard, N. & Oreffo, R. O. Harnessing nanotopography and integrin-matrix interactions to influence stem cell fate. Nat. Mater. 13, 558–569 (2014).
Elosegui-Artola, A. et al. Mechanical regulation of a molecular clutch defines force transmission and transduction in response to matrix rigidity. Nat. Cell Biol. 18, 540–548 (2016).
Bennett, M. et al. Molecular clutch drives cell response to surface viscosity. Proc. Natl Acad. Sci. USA 115, 1192–1197 (2018).
Malmstrom, J. et al. Focal complex maturation and bridging on 200 nm vitronectin but not fibronectin patches reveal different mechanisms of focal adhesion formation. Nano Lett. 11, 2264–2271 (2011).
Elosegui-Artola, A. et al. Force triggers YAP nuclear entry by regulating transport across nuclear pores. Cell 171, 1397–1410.e14 (2017).
Kim, J.-M. et al. The ERK MAPK pathway is essential for skeletal development and homeostasis. Int. J. Mol. Sci. 20, 1803 (2019).
Ge, C. et al. Reciprocal control of osteogenic and adipogenic differentiation by ERK/MAP kinase phosphorylation of Runx2 and PPARγ transcription factors. J. Cell Physiol. 231, 587–596 (2016).
Chen, Y. et al. Beta-catenin signaling pathway is crucial for bone morphogenetic protein 2 to induce new bone formation. J. Biol. Chem. 282, 526–533 (2007).
Hou, W. W., Zhu, Z. L., Zhou, Y., Zhang, C. X. & Yu, H. Y. Involvement of Wnt activation in the micromechanical vibration-enhanced osteogenic response of osteoblasts. J. Orthopaedic Sci. 16, 598–605 (2011).
Miyazaki, A. et al. Coordination of WNT signaling and ciliogenesis during odontogenesis by piezo type mechanosensitive ion channel component 1. Sci. Rep. 9, 14762 (2019).
Liu, Y. et al. Hydrogen sulfide maintains mesenchymal stem cell function and bone homeostasis via regulation of Ca2+ channel sulfhydration. Cell Stem Cell 15, 66–78 (2014).
Gaur, T. et al. Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J. Biol. Chem. 280, 33132–33140 (2005).
Choi, Y. et al. Sound waves induce neural differentiation of human bone marrow-derived mesenchymal stem cells via ryanodine receptor-induced calcium release and Pyk2 activation. Appl. Biochem. Biotechnol. 180, 682–694 (2016).
Coste, B. et al. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature 483, 176–181 (2012).
Kefauver, J. M., Ward, A. B. & Patapoutian, A. Discoveries in structure and physiology of mechanically activated ion channels. Nature 587, 567–576 (2020).
Li, Y. H. et al. Crosstalk between the COX2–PGE2–EP4 signaling pathway and primary cilia in osteoblasts after mechanical stimulation. J. Cell Physiol. 236, 4764–4777 (2021).
Loi, F. et al. Inflammation, fracture and bone repair. Bone 86, 119–130 (2016).
Ranzinger, J. et al. Nanoscale arrangement of apoptotic ligands reveals a demand for a minimal lateral distance for efficient death receptor activation. Nano Lett. 9, 4240–4245 (2009).
Varum, S. et al. Energy metabolism in human pluripotent stem cells and their differentiated counterparts. PLoS ONE 6, e20914 (2011).
Varum, S. et al. Enhancement of human embryonic stem cell pluripotency through inhibition of the mitochondrial respiratory chain. Stem Cell Res. 3, 142–156 (2009).
Marín-Cascales, E. et al. Whole-body vibration training and bone health in postmenopausal women: a systematic review and meta-analysis. Medicine 97, e11918 (2018).
Leighton, R., Phillips, M., Bhandari, M. & Zura, R. Low intensity pulsed ultrasound (LIPUS) use for the management of instrumented, infected, and fragility non-unions: a systematic review and meta-analysis of healing proportions. BMC Musculoskelet. Disord. 22, 1–9 (2021).
Auersperg, V. & Trieb, K. Extracorporeal shock wave therapy: an update. EFORT Open. Rev. 5, 584–592 (2020).
Köllmer, M., Buhrman, J. S., Zhang, Y. & Gemeinhart, R. A. Markers are shared between adipogenic and osteogenic differentiated mesenchymal stem cells. J. Dev. Biol. Tissue Eng. 5, 18 (2013).
Pel, J. et al. Platform accelerations of three different whole-body vibration devices and the transmission of vertical vibrations to the lower limbs. Med. Eng. Phys. 31, 937–944 (2009).
Rehn, B., Lidström, J., Skoglund, J. & Lindström, B. Effects on leg muscular performance from whole‐body vibration exercise: a systematic review. Scand. J. Med. Sci. Sports 17, 2–11 (2007).
Sitjà-Rabert, M. et al. Efficacy of whole body vibration exercise in older people: a systematic review. Disabil. Rehabil. 34, 883–893 (2012).
Chanou, K., Gerodimos, V., Karatrantou, K. & Jamurtas, A. Whole-body vibration and rehabilitation of chronic diseases: a review of the literature. J. Sports Sci. Med. 11, 187 (2012).
Cardinale, M. & Wakeling, J. Whole body vibration exercise: are vibrations good for you? Br. J. Sports Med. 39, 585–589 (2005).
Fischer, M. et al. Long-term effects of whole-body vibration on human gait: a systematic review and meta-analysis. Front. Neurol. 10, 627 (2019).
Slatkovska, L., Alibhai, S., Beyene, J. & Cheung, A. Effect of whole-body vibration on BMD: a systematic review and meta-analysis. Osteoporos. Int. 21, 1969–1980 (2010).
DadeMatthews, O. O. et al. Systematic review and meta-analyses on the effects of whole-body vibration on bone health. Complement. Ther. Med. 65, 102811 (2022).
Peretti, A. L., Ciqueleiro, R. T., Flores, L. J. F. & Bertolini, G. R. F. Use of whole-body vibration as osteoporosis treatment in postmenopausal women: a systematic review. Eur. J. Clin. Exp. Med. 17, 146–152 (2019).
Leung, K. S. et al. Low‐magnitude high‐frequency vibration accelerates callus formation, mineralization, and fracture healing in rats. J. Orthop. Res. 27, 458–465 (2009).
Shi, H.-F., Cheung, W.-H., Qin, L., Leung, A. H.-C. & Leung, K.-S. Low-magnitude high-frequency vibration treatment augments fracture healing in ovariectomy-induced osteoporotic bone. Bone 46, 1299–1305 (2010).
Wong, R. M. Y. et al. Fibrinolysis as a target to enhance osteoporotic fracture healing by vibration therapy in a metaphyseal fracture model. Bone Jt Res. 10, 41–50 (2021).
Chow, S. et al. Mechanical stimulation enhanced estrogen receptor expression and callus formation in diaphyseal long bone fracture healing in ovariectomy-induced osteoporotic rats. Osteoporos. Int. 27, 2989–3000 (2016).
Haffner-Luntzer, M., Lackner, I., Liedert, A., Fischer, V. & Ignatius, A. Effects of low-magnitude high-frequency vibration on osteoblasts are dependent on estrogen receptor α signaling and cytoskeletal remodeling. Biochem. Biophys. Res. Commun. 503, 2678–2684 (2018).
Chow, S. et al. Vibration treatment modulates macrophage polarisation and enhances early inflammatory response in oestrogen-deficient osteoporotic-fracture healing. Eur. Cell Mater. 38, 228–245 (2019).
Jawed, Y., Beli, E., March, K., Kaleth, A. & Loghmani, M. T. Whole-body vibration training increases stem/progenitor cell circulation levels and may attenuate inflammation. Military Med. 185, 404–412 (2020).
Jing, D. et al. Effect of low-level mechanical vibration on osteogenesis and osseointegration of porous titanium implants in the repair of long bone defects. Sci. Rep. 5, 17134 (2015).
Wang, J. et al. Vibration and β‐hydroxy‐β‐methylbutyrate treatment suppresses intramuscular fat infiltration and adipogenic differentiation in sarcopenic mice. J. Cachexia, Sarcopenia Muscle 11, 564–577 (2020).
Judex, S. & Rubin, C. Is bone formation induced by high-frequency mechanical signals modulated by muscle activity? J. Musculoskelet. Neuronal Interact. 10, 3 (2010).
Seo, B. R. et al. Skeletal muscle regeneration with robotic actuation–mediated clearance of neutrophils. Sci. Transl. Med. 13, eabe8868 (2021).
Lara-Castillo, N. et al. In vivo mechanical loading rapidly activates β-catenin signaling in osteocytes through a prostaglandin mediated mechanism. Bone 76, 58–66 (2015).
Liu, C. et al. Effects of mechanical loading on cortical defect repair using a novel mechanobiological model of bone healing. Bone 108, 145–155 (2018).
Birmingham, E. et al. Mechanical stimulation of bone marrow in situ induces bone formation in trabecular explants. Ann. Biomed. Eng. 43, 1036–1050 (2015).
Zhao, C., Liu, H., Tian, C., Zhang, C. & Wang, W. Multi-scale numerical simulation on mechano-transduction of osteocytes in different gravity fields. Comput. Meth. Biomech. Biomed. Eng. 26, 1419–1430 (2023).
Williams, J. A. et al. Developing and investigating a nanovibration intervention for the prevention/reversal of bone loss following spinal cord injury. ACS Nano 18, 17630–17641 (2024).
McKnight, C. L., Doman, D. A., Brown, J. A., Bance, M. & Adamson, R. B. Direct measurement of the wavelength of sound waves in the human skull. J. Acoust. Soc. Am. 133, 136–145 (2013).
McLeod, R., Roberts, W., Perry, I., Richardson, B. & Culling, J. Scanning laser Doppler vibrometry of the cranium when stimulated by a B71 bone transducer. Appl. Acoust. 142, 53–58 (2018).
Dobrev, I. et al. Sound wave propagation on the human skull surface with bone conduction stimulation. Hearing Res. 355, 1–13 (2017).
Busse, J. W. et al. Re-evaluation of low intensity pulsed ultrasound in treatment of tibial fractures (TRUST): randomized clinical trial. BMJ 355, i5351 (2016).
Poolman, R. W. et al. Low intensity pulsed ultrasound (LIPUS) for bone healing: a clinical practice guideline. BMJ 356, j576 (2017).
Dolgin, E. Sizzling interest in lab-grown meat belies lack of basic research. Nature 566, 161–163 (2019).
Stout, A. J. et al. Simple and effective serum-free medium for sustained expansion of bovine satellite cells for cell cultured meat. Commun. Biol. 5, 466 (2022).
Coords, M. et al. The effects of low-intensity pulsed ultrasound upon diabetic fracture healing. J. Orthop. Res. 29, 181–188 (2011).
Curtis, A. S. et al. Cell interactions at the nanoscale: piezoelectric stimulation. IEEE Trans. Nanobiosci. 12, 247–254 (2013).
Karimi, E. et al. Nanoscale vibration could promote tenogenic differentiation of umbilical cord mesenchymal stem cells. In Vitro Cell. Dev. Biol. Anim. 59, 401–409 (2023).
Kim, I. S., Song, Y. M., Lee, B. & Hwang, S. J. Human mesenchymal stromal cells are mechanosensitive to vibration stimuli. J. Dent. Res. 91, 1135–1140 (2012).
Demiray, L. & Ozcivici, E. Bone marrow stem cells adapt to low-magnitude vibrations by altering their cytoskeleton during quiescence and osteogenesis. Turk. J. Biol. 39, 88–97 (2015).
Pravitharangul, A., Suttapreyasri, S. & Leethanakul, C. Iliac and mandible osteoblasts exhibit varied responses to LMHF vibration. Cell Biol. Int. 42, 1349–1357 (2018).
Chen, B. et al. Low-magnitude, high-frequency vibration promotes the adhesion and the osteogenic differentiation of bone marrow-derived mesenchymal stem cells cultured on a hydroxyapatite-coated surface: The direct role of Wnt/β-catenin signaling pathway activation. Int. J. Mol. Med. 38, 1531–1540 (2016).
Macione, J. et al. Stimulation of osteoblast differentiation with guided ultrasound waves. J. Ther. Ultrasound 3, 12 (2015).
Chu, Y. C., Lim, J., Hwang, W. H., Lin, Y. X. & Wang, J. L. Piezoelectric stimulation by ultrasound facilitates chondrogenesis of mesenchymal stem cells. J. Acoust. Soc. Am. 148, El58 (2020).
Hortobagyi, D. et al. In vitro mechanical vibration down-regulates pro-inflammatory and pro-fibrotic signaling in human vocal fold fibroblasts. PLoS ONE 15, e0241901 (2020).
García-López, S., Villanueva, R. E., Massó-Rojas, F., Páez-Arenas, A. & Meikle, M. C. Micro-vibrations at 30 Hz on bone cells cultivated in vitro produce soluble factors for osteoclast inhibition and osteoblast activity. Arch. Oral. Biol. 110, 104594 (2020).
Sun, T. et al. Effects of mechanical vibration on cell morphology, proliferation, apoptosis, and cytokine expression/secretion in osteocyte-like MLO-Y4 cells exposed to high glucose. Cell Biol. Int. 44, 216–228 (2019).
Wang, C.-Z. et al. Low-magnitude vertical vibration enhances myotube formation in C2C12 myoblasts. J. Appl. Physiol. 109, 840–848 (2010).
Lin, Y. H. et al. The essential role of stathmin in myoblast C2C12 for vertical vibration-induced myotube formation. Biomolecules 11, 1583 (2021).
Choi, Y. K., Cho, H., Seo, Y. K., Yoon, H. H. & Park, J. K. Stimulation of sub-sonic vibration promotes the differentiation of adipose tissue-derived mesenchymal stem cells into neural cells. Life Sci. 91, 329–337 (2012).
Cho, H., Seo, Y.-K., Yoon, H.-H., Choi, Y.-K. & Park, J.-K. Neural differentiation of umbilical cord mesenchymal stem cells by sub-sonic vibration. Life Sci. 90, 591–599 (2012).
Cho, H., Park, H. J. & Seo, Y. K. Induction of PLXNA4 gene during neural differentiation in human umbilical-cord-derived mesenchymal stem cells by low-intensity sub-sonic vibration. Int. J. Mol. Sci. 23, 1522 (2022).
Benjakul, S., Leethanakul, C. & Jitpukdeebodintra, S. Low magnitude high frequency vibration induces RANKL via cyclooxygenase pathway in human periodontal ligament cells in vitro. J. Oral. Biol. Craniofac. Res. 9, 251–255 (2019).
Ye, M. et al. Vibration induces BAFF overexpression and aberrant O-glycosylation of IgA1 in cultured human tonsillar mononuclear cells in IgA nephropathy. Biomed. Res. Int. 2016, 9125960 (2016).
Touchstone, H. et al. Recovery of stem cell proliferation by low intensity vibration under simulated microgravity requires LINC complex. npj Microgravity 5, 11 (2019).
Robertson, S. N. et al. Reduction of Pseudomonas aeruginosa biofilm formation through the application of nanoscale vibration. J. Biosci. Bioeng. 129, 379–386 (2020).
Tanaka, S. M. et al. Effects of broad frequency vibration on cultured osteoblasts. J. Biomech. 36, 73–80 (2003).
Ballikaya, S. et al. Process data of allogeneic ex vivo-expanded ABCB5+ mesenchymal stromal cells for human use: off-the-shelf GMP-manufactured donor-independent ATMP. Stem Cell Res. Ther. 11, 482 (2020).
Simaria, A. S. et al. Allogeneic cell therapy bioprocess economics and optimization: single‐use cell expansion technologies. Biotechnol. Bioeng. 111, 69–83 (2014).
Lawson, T. et al. Process development for expansion of human mesenchymal stromal cells in a 50L single-use stirred tank bioreactor. Biochem. Eng. J. 120, 49–62 (2017).
Karnieli, O. et al. A consensus introduction to serum replacements and serum-free media for cellular therapies. Cytotherapy 19, 155–169 (2017).
Acknowledgements
We thank EPSRC for grants EP/N013905/1 and EP/P001114/1.
Author information
Authors and Affiliations
Contributions
O.J.-L., P.G.C. and M.J.D. led the writing of the review, with all authors involved in contributing to different sections. All authors critically read the review.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Bioengineering thanks Benoit Ladoux, Sylvain Gabriele, Marine Luciano and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
The failure of a phase IIb trial of ALLOB: https://www.fiercebiotech.com/biotech/bone-therapeutics-final-asset-buried-after-failure-phase-2-fracture-study
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Johnson-Love, O., Salmeron-Sanchez, M., Reid, S. et al. Vibration-based cell engineering. Nat Rev Bioeng 3, 408–429 (2025). https://doi.org/10.1038/s44222-025-00273-x
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44222-025-00273-x
This article is cited by
-
Nonlinear electromechanical coupling dynamics of a two-degree-of-freedom hybrid energy harvester
Applied Mathematics and Mechanics (2025)