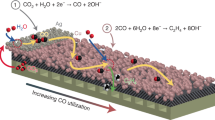
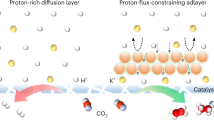
Abstract
Carbon dioxide (CO2) valorization is a promising pathway for mitigating greenhouse gas emissions from the chemical sector and reducing the reliance of chemical manufacturing on fossil fuel feedstocks. This Perspective discusses tandem catalytic paradigms for sustainable CO2 conversion that have potential advantages over processes using single-functional catalysts. Recent progress is discussed for tandem catalysis using multifunctional catalysts in a single reactor, as well as tandem reactors involving multiple catalysts. Opportunities for further developing these tandem strategies for thermochemical and electrochemical processes in various configurations are presented to encourage research in this burgeoning field.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Technology Roadmap—Energy and GHG Reductions in the Chemical Industry via Catalytic Processes (IEA, 2013); https://www.iea.org/reports/technology-roadmap-energy-and-ghg-reductions-in-the-chemical-industry-via-catalytic-processes
The Future of Petrochemicals (IEA, 2018); https://www.iea.org/reports/the-future-of-petrochemicals
Ritchie, H., Roser, M. & Rosado, P. CO2 and greenhouse gas emissions. Our World In Data https://ourworldindata.org/co2-and-greenhouse-gas-emissions (2020).
Biswas, A. N., Winter, L. R., Xie, Z. & Chen, J. G. Utilizing CO2 as a reactant for C3 oxygenate production via tandem reactions. JACS Au 3, 293–305 (2023).
Tackett, B. M., Gomez, E. & Chen, J. G. Net reduction of CO2 via its thermocatalytic and electrocatalytic transformation reactions in standard and hybrid processes. Nat. Catal. 2, 381–386 (2019).
Overa, S., Feric, T. G., Park, A.-H. A. & Jiao, F. Tandem and hybrid processes for carbon dioxide utilization. Joule 5, 8–13 (2021).
Zheng, W. et al. Designs of tandem catalysts and cascade catalytic systems for CO2 upgrading. Angew. Chem. Int. Ed. 62, e202307283 (2023).
Gioria, E. et al. Rational design of tandem catalysts using a core–shell structure approach. Nanoscale Adv. 3, 3454–3459 (2021).
Jin, K. et al. Conversion of CO2 to gasoline over tandem Fe/C and HZSM-5 catalysts. Sustain. Energy Fuels 7, 1265–1272 (2023).
Xie, C. et al. Tandem catalysis for CO2 hydrogenation to C2–C4 hydrocarbons. Nano Lett. 17, 3798–3802 (2017).
Gomez, E., Nie, X., Lee, J. H., Xie, Z. & Chen, J. G. Tandem reactions of CO2 reduction and ethane aromatization. J. Am. Chem. Soc. 141, 17771–17782 (2019).
Xu, D., Yang, H., Hong, X., Liu, G. & Edman Tsang, S. C. Tandem catalysis of direct CO2 hydrogenation to higher alcohols. ACS Catal. 11, 8978–8984 (2021).
Li, Z. et al. Highly selective conversion of carbon dioxide to aromatics over tandem catalysts. Joule 3, 570–583 (2019).
Gao, P. et al. Direct conversion of CO2 into liquid fuels with high selectivity over a bifunctional catalyst. Nat. Chem. 9, 1019–1024 (2017).
Wei, X., Li, Y., Hua, Z., Chen, L. & Shi, J. One-pot synthesized nickel-doped hierarchically porous beta zeolite for enhanced methanol electrocatalytic oxidation activity. ChemCatChem 12, 6285–6290 (2020).
Xie, Z. et al. Reactions of CO2 and ethane enable CO bond insertion for production of C3 oxygenates. Nat. Commun. 11, 1887 (2020).
Saad, J. M. & Williams, P. T. Pyrolysis-catalytic-dry reforming of waste plastics and mixed waste plastics for syngas production. Energy Fuels 30, 3198–3204 (2016).
Iyengar, P., Kolb, M. J., Pankhurst, J., Calle-Vallejo, F. & Buonsanti, R. Theory-guided enhancement of CO2 reduction to ethanol on Ag–Cu tandem catalysts via particle-size effects. ACS Catal. 11, 13330–13336 (2021).
Xiong, W. et al. Morphology and composition dependence of multicomponent Cu-based nanoreactor for tandem electrocatalysis CO2 reduction. Appl. Catal. B 314, 121498 (2022).
Nitopi, S. et al. Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte. Chem. Rev. 119, 7610–7672 (2019).
Ren, D., Ang, B. S.-H. & Yeo, B. S. Tuning the selectivity of carbon dioxide electroreduction toward ethanol on oxide-derived CuxZn catalysts. ACS Catal. 6, 8239–8247 (2016).
Lee, C. W. et al. Defining a materials database for the design of copper binary alloy catalysts for electrochemical CO2 conversion. Adv. Mater. 30, 1704717 (2018).
Chen, C. et al. Cu–Ag tandem catalysts for high-rate CO2 electrolysis toward multicarbons. Joule 4, 1688–1699 (2020).
Lin, Y.-R. et al. Vapor-fed electrolyzers for carbon dioxide reduction using tandem electrocatalysts: cuprous oxide coupled with nickel-coordinated nitrogen-doped carbon. Adv. Funct. Mater. 32, 2113252 (2022).
Guzmán, H. et al. CO2 conversion to alcohols over Cu/ZnO catalysts: prospective synergies between electrocatalytic and thermocatalytic routes. ACS Appl. Mater. Interfaces 14, 517–530 (2022).
Morales-Guio, C. G. et al. Improved CO2 reduction activity towards C2+ alcohols on a tandem gold on copper electrocatalyst. Nat. Catal. 1, 764–771 (2018).
Akter, T., Pan, H. & Barile, C. J. Tandem electrocatalytic CO2 reduction inside a membrane with enhanced selectivity for ethylene. J. Phys. Chem. C 126, 10045–10052 (2022).
Zhang, T. et al. Highly selective and productive reduction of carbon dioxide to multicarbon products via in situ CO management using segmented tandem electrodes. Nat. Catal. 5, 202–211 (2022).
Cao, B., Li, F.-Z. & Gu, J. Designing Cu-based tandem catalysts for CO2 electroreduction based on mass transport of CO intermediate. ACS Catal. https://doi.org/10.1021/acscatal.2c02579 (2022).
Lum, Y. & Ager, J. W. Sequential catalysis controls selectivity in electrochemical CO2 reduction on Cu. Energy Environ. Sci. 11, 2935–2944 (2018).
Zhang, T., Li, Z., Ummireddi, A. K. & Wu, J. Navigating CO utilization in tandem electrocatalysis of CO2. Trends Chem. 5, 252–266 (2023).
Yin, Z. et al. Hybrid catalyst coupling single-atom Ni and nanoscale Cu for efficient CO2 electroreduction to ethylene. J. Am. Chem. Soc. 144, 20931–20938 (2022).
De Luna, P. et al. Metal–organic framework thin films on high-curvature nanostructures toward tandem electrocatalysis. ACS Appl. Mater. Interfaces 10, 31225–31232 (2018).
Cheng, N., Ren, L., Xu, X., Du, Y. & Dou, S. X. Recent development of zeolitic imidazolate frameworks (ZIFs) derived porous carbon based materials as electrocatalysts. Adv. Energy Mater. 8, 1801257 (2018).
Vasić, M. et al. Efficient hydrogen evolution electrocatalysis in alkaline medium using Pd-modified zeolite X. Electrochim. Acta 259, 882–892 (2018).
Jeng, E. & Jiao, F. Investigation of CO2 single-pass conversion in a flow electrolyzer. React. Chem. Eng. 5, 1768–1775 (2020).
Jouny, M., Hutchings, G. S. & Jiao, F. Carbon monoxide electroreduction as an emerging platform for carbon utilization. Nat. Catal. 2, 1062–1070 (2019).
Wang, X. et al. Efficient electrosynthesis of n-propanol from carbon monoxide using a Ag–Ru–Cu catalyst. Nat. Energy 7, 170–176 (2022).
Wang, X. et al. Efficient upgrading of CO to C3fuel using asymmetric C–C coupling active sites. Nat. Commun. 10, 5186 (2019).
Hann, E. C. et al. A hybrid inorganic–biological artificial photosynthesis system for energy-efficient food production. Nat. Food 3, 461–471 (2022).
Jouny, M. et al. Formation of carbon–nitrogen bonds in carbon monoxide electrolysis. Nat. Chem. 11, 846–851 (2019).
Garg, S., Biswas, A. N. & Chen, J. G. Opportunities for CO2 upgrading to C3 oxygenates using tandem electrocatalytic-thermocatalytic processes. Carbon Future 1, 9200002 (2023).
Tackett, B. M., Lee, J. H. & Chen, J. G. Electrochemical conversion of CO2 to syngas with palladium-based electrocatalysts. Acc. Chem. Res. 53, 1535–1544 (2020).
Uslamin, E. A. et al. Aromatization of ethylene over zeolite-based catalysts. Catal. Sci. Technol. 10, 2774–2785 (2020).
Bonnin, A. et al. Mechanisms of aromatization of dilute ethylene on HZSM-5 and on Zn/HZSM-5 catalysts. Appl. Catal. Gen. 611, 117974 (2021).
Biswas, A. N. et al. Tandem electrocatalytic–thermocatalytic reaction scheme for CO2 conversion to C3 oxygenates. ACS Energy Lett. 7, 2904–2910 (2022).
Lee, M. G. et al. Selective synthesis of butane from carbon monoxide using cascade electrolysis and thermocatalysis at ambient conditions. Nat. Catal. https://doi.org/10.1038/s41929-023-00937-0 (2023).
Ponsard, L. et al. Coupling electrocatalytic CO2 reduction with thermocatalysis enables the formation of a lactone monomer. ChemSusChem 14, 2198–2204 (2021).
Li, Z. et al. Recent advances in process and catalyst for CO2 reforming of methane. Renew. Sustain. Energy Rev. 134, 110312 (2020).
Pham, T. T. P. et al. Microwave-assisted dry reforming of methane for syngas production: a review. Environ. Chem. Lett. 18, 1987–2019 (2020).
Xie, Z. et al. CO2 fixation into carbon nanofibres using electrochemical–thermochemical tandem catalysis. Nat. Catal. https://doi.org/10.1038/s41929-023-01085-1 (2024).
Küngas, R. Electrochemical CO2 reduction for CO production: comparison of low- and high-temperature electrolysis technologies. J. Electrochem. Soc. 167, 044508 (2020).
Song, Y. et al. High-temperature CO2 electrolysis in solid oxide electrolysis cells: developments, challenges, and prospects. Adv. Mater. 31, 1902033 (2019).
Wang, Y., Liu, T., Lei, L. & Chen, F. High temperature solid oxide H2O/CO2 co-electrolysis for syngas production. Fuel Process. Technol. 161, 248–258 (2017).
Yuan, L., Qi, M.-Y., Tang, Z.-R. & Xu, Y.-J. Coupling strategy for CO2 valorization integrated with organic synthesis by heterogeneous photocatalysis. Angew. Chem. Int. Ed. 60, 21150–21172 (2021).
Xia, Y.-S. et al. Tandem utilization of CO2 photoreduction products for the carbonylation of aryl iodides. Nat. Commun. 13, 2964 (2022).
Zhang, L. et al. Direct coupling of thermo- and photocatalysis for conversion of CO2–H2O into fuels. ChemSusChem 10, 4709–4714 (2017).
Crandall, B. S., Overa, S., Shin, H. & Jiao, F. Turning carbon dioxide into sustainable food and chemicals: how electrosynthesized acetate is paving the way for fermentation innovation. Acc. Chem. Res. 56, 1505–1516 (2023).
Martín-Espejo, J. L., Gandara-Loe, J., Odriozola, J. A., Reina, T. R. & Pastor-Pérez, L. Sustainable routes for acetic acid production: traditional processes vs a low-carbon, biogas-based strategy. Sci. Total Environ. 840, 156663 (2022).
Budsberg, E., Morales-Vera, R., Crawford, J. T., Bura, R. & Gustafson, R. Production routes to bio-acetic acid: life cycle assessment. Biotechnol. Biofuels 13, 154 (2020).
Ji, Y. et al. Selective CO-to-acetate electroreduction via intermediate adsorption tuning on ordered Cu–Pd sites. Nat. Catal. 5, 251–258 (2022).
Kutscha, R. & Pflügl, S. Microbial upgrading of acetate into value-added products—examining microbial diversity, bioenergetic constraints and metabolic engineering approaches. Int. J. Mol. Sci. 21, 8777 (2020).
Sun, S., Ding, Y., Liu, M., Xian, M. & Zhao, G. Comparison of glucose, acetate and ethanol as carbon resource for production of poly(3-hydroxybutyrate) and other acetyl-CoA derivatives. Front. Bioeng. Biotechnol. 8, 833 (2020).
Gong, G. et al. Metabolic engineering using acetate as a promising building block for the production of bio‐based chemicals. Eng. Microbiol. 2, 100036 (2022).
Haas, T., Krause, R., Weber, R., Demler, M. & Schmid, G. Technical photosynthesis involving CO2 electrolysis and fermentation. Nat. Catal. 1, 32–39 (2018).
Luc, W., Jouny, M., Rosen, J. & Jiao, F. Carbon dioxide splitting using an electro-thermochemical hybrid looping strategy. Energy Environ. Sci. 11, 2928–2934 (2018).
Bajracharya, S. et al. Biotransformation of carbon dioxide in bioelectrochemical systems: state of the art and future prospects. J. Power Sources 356, 256–273 (2017).
Liu, S., Winter, L. R. & Chen, J. G. Review of plasma-assisted catalysis for selective generation of oxygenates from CO2 and CH4. ACS Catal. 10, 2855–2871 (2020).
Bogaerts, A. & Neyts, E. C. Plasma technology: an emerging technology for energy storage. ACS Energy Lett. 3, 1013–1027 (2018).
Biswas, A. N. et al. Oxygenate production from plasma-activated reaction of CO2 and ethane. ACS Energy Lett. 7, 236–241 (2022).
Gómez-Ramírez, A., Rico, V. J., Cotrino, J., González-Elipe, A. R. & Lambert, R. M. Low temperature production of formaldehyde from carbon dioxide and ethane by plasma-assisted catalysis in a ferroelectrically moderated dielectric barrier discharge reactor. ACS Catal. 4, 402–408 (2014).
Acknowledgements
We acknowledge support by the US Department of Energy, Office of Basic Energy Sciences, Catalysis Science Program (grant no. DE-FG02-13ER16381 and contract no. DE-SC0012704). S.G. acknowledges support by the National Science Foundation Graduate Research Fellowship under grant no. DGE-2036197.
Author information
Authors and Affiliations
Contributions
S.G., Z.X. and J.G.C. conceived the concept of this paper. All authors participated in writing the paper. J.G.C. and Z.X. supervised the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Engineering thanks Yizhi Xiang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Garg, S., Xie, Z. & Chen, J.G. Tandem reactors and reactions for CO2 conversion. Nat Chem Eng 1, 139–148 (2024). https://doi.org/10.1038/s44286-023-00020-2
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44286-023-00020-2
This article is cited by
-
Biogas sequestration to carbon nanofibers via tandem catalytic strategies
Nature Chemical Engineering (2025)
-
Transformation of CO2 to C2+ alcohols by tailoring the oxygen bonding via Fe-based tandem catalyst
Nature Communications (2025)
-
Active learning-guided catalyst design for selective acetate production in CO electroreduction
Nature Communications (2025)
-
Getting rid of CO2 for good
Nature Chemical Engineering (2025)
-
Ethane dehydrogenation over CaCO3-mediated tandem catalysts
Nature Communications (2025)