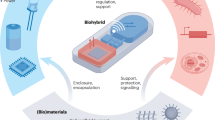
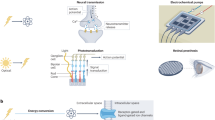
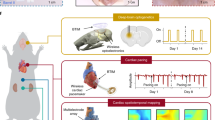
Abstract
Self-sustainable bioelectronic devices that incorporate physiological synchronization functions are attracting increasing research interest because they could provide the variable functions required by living cells and tissues. However, from the popular viewpoint, self-sustainable bioelectronic devices are presently regarded to provide unidirectional stimulation, similarly to traditional bioelectronic devices that prompt cells and tissues to passively respond to the electrical cues delivered to them. The active effect of self-sustainable bioelectronic devices, which allows cells and/or tissues to autonomously alter the delivered electrical stimulation on demand, has not been fully recognized. This Perspective article presents the insight that self-sustainable bioelectronics could act as a bidirectional ‘bridge’ linking the electrical modulation of a cell or tissue with its growth and development requirements, thereby establishing a fully autonomous, closed-loop regulatory system. The interaction processes arising in microscopic (cell–piezoelectric material) and macroscopic (organ–electromechanically coupled device) systems are discussed, and typical examples of self-sustainable bioelectronics are presented, highlighting the key challenges of signal fidelity and long-term device stability. Predictions of the future trajectory of self-sustainable bioelectronics, and design considerations for the next generation of intelligent bioelectronic devices, are also included.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Fukui, H. et al. Bioelectric signaling and the control of cardiac cell identity in response to mechanical forces. Science 374, 351–354 (2021).
Levin, M. Bioelectric signaling: reprogrammable circuits underlying embryogenesis, regeneration, and cancer. Cell 184, 1971–1989 (2021).
Payne, S. C., Furness, J. B. & Stebbing, M. J. Bioelectric neuromodulation for gastrointestinal disorders: effectiveness and mechanisms. Nat. Rev. Gastroenterol. Hepatol. 16, 89–105 (2019).
Liu, Z., Wan, X., Wang, Z. L. & Li, L. Electroactive biomaterials and systems for cell fate determination and tissue regeneration: design and applications. Adv. Mater. 33, e2007429 (2021).
Gong, S., Lu, Y., Yin, J., Levin, A. & Cheng, W. Materials-driven soft wearable bioelectronics for connected healthcare. Chem. Rev. 124, 455–553 (2024).
Li, T. et al. Soft ferroelectret ultrasound receiver for targeted peripheral neuromodulation. Nat. Commun. 14, 8386 (2023).
Bhatia, A. et al. Wireless battery-free and fully implantable organ interfaces. Chem. Rev. 124, 2205–2280 (2024).
Lee, Y. C. et al. The dynamic roles of the bladder tumour microenvironment. Nat. Rev. Urol. 19, 515–533 (2022).
Mata, R. et al. The dynamic inflammatory tissue microenvironment: signality and disease therapy by biomaterials. Research 2021, 4189516 (2021).
Huang, G. et al. Functional and biomimetic materials for engineering of the three-dimensional cell microenvironment. Chem. Rev. 117, 12764–12850 (2017).
Gilron, R. et al. Long-term wireless streaming of neural recordings for circuit discovery and adaptive stimulation in individuals with Parkinson’s disease. Nat. Biotechnol. 39, 1078–1085 (2021).
Jin, F. et al. Biofeedback electrostimulation for bionic and long-lasting neural modulation. Nat. Commun. 13, 5302 (2022).
Jin, F. et al. Physiologically self-regulated, fully implantable, battery-free system for peripheral nerve restoration. Adv. Mater. 33, e2104175 (2021).
Li, J. et al. Biomaterials and bioelectronics for self-powered neurostimulation. Biomaterials 304, 122421 (2024).
Xiao, X., Chen, G., Libanori, A. & Chen, J. Wearable triboelectric nanogenerators for therapeutics. Trends Chem. 3, 279–290 (2021).
Panda, S. et al. Piezoelectric energy harvesting systems for biomedical applications. Nano Energy 100, 107514 (2022).
Kapat, K., Shubhra, Q. T. H., Zhou, M. & Leeuwenburgh, S. Piezoelectric nano‐biomaterials for biomedicine and tissue regeneration. Adv. Funct. Mater. 30, 1909045 (2020).
Long, Y., Li, J., Yang, F., Wang, J. & Wang, X. Wearable and implantable electroceuticals for therapeutic electrostimulations. Adv. Sci. 8, 2004023 (2021).
Zhou, L. et al. Advances in applications of piezoelectronic electrons in cell regulation and tissue regeneration. J. Mater. Chem. B 10, 8797–8823 (2022).
Murillo, G. et al. Electromechanical nanogenerator–cell interaction modulates cell activity. Adv. Mater. 29, 1605048 (2017).
Nain, A. et al. Progress in the development of piezoelectric biomaterials for tissue remodeling. Biomaterials 307, 122528 (2024).
Guo, H. et al. Cell activity manipulation through optimizing piezoelectricity and polarization of diphenylalanine peptide nanotube-based nanocomposite. Chem. Eng. J. 468, 143597 (2023).
Conta, G., Libanori, A., Tat, T., Chen, G. & Chen, J. Triboelectric nanogenerators for therapeutic electrical stimulation. Adv. Mater. 33, e2007502 (2021).
Yin, J., Wang, S., Tat, T. & Chen, J. Motion artefact management for soft bioelectronics. Nat. Rev. Bioeng. 2, 541–558 (2024).
Zhao, X. et al. Permanent fluidic magnets for liquid bioelectronics. Nat. Mater. 23, 703–710 (2024).
Elsanadidy, E. et al. Advances in triboelectric nanogenerators for self‐powered neuromodulation. Adv. Funct. Mater. 33, 2211177 (2023).
Xia, G., Song, B. & Fang, J. Electrical stimulation enabled via electrospun piezoelectric polymeric nanofibers for tissue regeneration. Research 2022, 9896274 (2022).
Liu, Z. et al. Cell-traction-triggered on-demand electrical stimulation for neuron-like differentiation. Adv. Mater. 33, e2106317 (2021).
Wu, H. et al. Stem cell self‐triggered regulation and differentiation on polyvinylidene fluoride electrospun nanofibers. Adv. Funct. Mater. 34, 2309270 (2023).
Ren, J. et al. Piezoelectric dual network dressing with adaptive electrical stimulation for diabetic infected wound repair via antibacterial, antioxidant, anti-inflammation, and angiogenesis. Chem. Eng. J. 491, 151801 (2024).
Zhang, H. Y. et al. Biodegradable ferroelectric molecular crystal with large piezoelectric response. Science 383, 1492–1498 (2024).
Li, T. et al. Cell activity modulation and its specific function maintenance by bioinspired electromechanical nanogenerator. Sci. Adv. 7, eabh2350 (2021).
Zhang, L. et al. Recent progress on structure manipulation of poly(vinylidene fluoride)‐based ferroelectric polymers for enhanced piezoelectricity and applications. Adv. Funct. Mater. 33, 2301302 (2023).
Xu, Q. et al. Construction of bio-piezoelectric platforms: from structures and synthesis to applications. Adv. Mater. 33, e2008452 (2021).
Li, T. et al. High-performance poly(vinylidene difluoride)/dopamine core/shell piezoelectric nanofiber and its application for biomedical sensors. Adv. Mater. 33, e2006093 (2021).
Nguyen, T. D. et al. Piezoelectric nanoribbons for monitoring cellular deformations. Nat. Nanotechnol. 7, 587–593 (2012).
Zhang, X. et al. Piezoelectric nanotopography induced neuron-like differentiation of stem cells. Adv. Funct. Mater. 29, 1900372 (2019).
Li, J. et al. Functional material-mediated wireless physical stimulation for neuro-modulation and regeneration. J. Mater. Chem. B 11, 9056–9083 (2023).
Leijnse, N. et al. Filopodia rotate and coil by actively generating twist in their actin shaft. Nat. Commun. 13, 1636 (2022).
Kechagia, J. Z., Ivaska, J. & Roca-Cusachs, P. Integrins as biomechanical sensors of the microenvironment. Nat. Rev. Mol. Cell Biol. 20, 457–473 (2019).
Marques-Almeida, T., Fernandes, H. J. R., Lanceros-Mendez, S. & Ribeiro, C. Surface charge and dynamic mechanoelectrical stimuli improves adhesion, proliferation and differentiation of neuron-like cells. J. Mater. Chem. B 11, 144–153 (2022).
Di, X. et al. Cellular mechanotransduction in health and diseases: from molecular mechanism to therapeutic targets. Sig. Transduct. Target. Ther. 8, 282 (2023).
Du, H. et al. Tuning immunity through tissue mechanotransduction. Nat. Rev. Immunol. 23, 174–188 (2023).
Yuan, X. et al. Piezoelectricity, pyroelectricity, and ferroelectricity in biomaterials and biomedical applications. Adv. Mater. 36, 2308726 (2024).
Mao, R. et al. Piezoelectric stimulation from electrospun composite nanofibers for rapid peripheral nerve regeneration. Nano Energy 98, 107322 (2022).
Fernandez-Yague, M. A. et al. A self-powered piezo-bioelectric device regulates tendon repair-associated signaling pathways through modulation of mechanosensitive ion channels. Adv. Mater. 33, 2008788 (2021).
Liu, Y. et al. Exercise-induced piezoelectric stimulation for cartilage regeneration in rabbits. Sci. Transl. Med. 14, eabi7282 (2022).
Joo, S. et al. Piezoelectrically and topographically engineered scaffolds for accelerating bone regeneration. ACS Appl. Mater. Interfaces 16, 1999–2011 (2024).
Qian, L. et al. Self-adhesive and self-sustainable bioelectronic patch for physiological feedback electronic modulation of soft organs. Adv. Mater. 36, 2406636 (2024).
Luo, R., Dai, J., Zhang, J. & Li, Z. Accelerated skin wound healing by electrical stimulation. Adv. Healthc. Mater. 10, 2100557 (2021).
Yeh, C. C. et al. Timing of applying electrical stimulation is an important factor deciding the success rate and maturity of regenerating rat sciatic nerves. Neurorehab. Neural Repair 24, 730–735 (2010).
Wang, R., Sui, J. & Wang, X. Natural piezoelectric biomaterials: a biocompatible and sustainable building block for biomedical devices. ACS Nano 16, 17708–17728 (2022).
Zheng, W. et al. Interfacial polarization locked flexible β-phase glycine/Nb2CTx piezoelectric nanofibers. Small 20, 2308715 (2024).
Cheng, Y. et al. Boosting the piezoelectric sensitivity of amino acid crystals by mechanical annealing for the engineering of fully degradable force sensors. Adv. Sci. 10, 2207269 (2023).
Yang, F. et al. Wafer-scale heterostructured piezoelectric bio-organic thin films. Science 373, 337–342 (2021).
Zhang, Z. et al. Van der Waals exfoliation processed biopiezoelectric submucosa ultrathin films. Adv. Mater. 34, 2200864 (2022).
Xie, X. et al. Biomimetic nanofibrillar hydrogel with cell-adaptable network for enhancing cellular mechanotransduction, metabolic energetics, and bone regeneration. J. Am. Chem. Soc. 145, 15218–15229 (2023).
Zhang, Y. et al. Electroactive biomaterials synergizing with electrostimulation for cardiac tissue regeneration and function-monitoring. Mater. Today 70, 237–272 (2023).
Zhang, X. et al. Electrical stimulation system based on electroactive biomaterials for bone tissue engineering. Mater. Today 68, 177–203 (2023).
Schmitt, M. S. et al. Machine learning interpretable models of cell mechanics from protein images. Cell 187, 481–494 (2024).
Petsakou, A. & Perrimon, N. Bioelectric regulation of intestinal stem cells. Trends Cell Biol. 33, 555–567 (2023).
Sekitani, T. The disappearing boundary between organism and machine. Science 380, 690–691 (2023).
Wagner, F. B. et al. Targeted neurotechnology restores walking in humans with spinal cord injury. Nature 563, 65–71 (2018).
Nair, V. et al. Miniature battery-free bioelectronics. Science 382, eabn4732 (2023).
Tang, W., Sun, Q. & Wang, Z. L. Self-powered sensing in wearable electronics horizontal line — a paradigm shift technology. Chem. Rev. 123, 12105–12134 (2023).
Deng, W. et al. Piezoelectric nanogenerators for personalized healthcare. Chem. Soc. Rev. 51, 3380–3435 (2022).
Choi, D. et al. Recent advances in triboelectric nanogenerators: from technological progress to commercial applications. ACS Nano 17, 11087–11219 (2023).
Kang, M. et al. Advances in bioresorbable triboelectric nanogenerators. Chem. Rev. 123, 11559–11618 (2023).
Zhou, Y. et al. Giant magnetoelastic effect in soft systems for bioelectronics. Nat. Mater. 20, 1670–1676 (2021).
Chen, G. et al. Discovering giant magnetoelasticity in soft matter for electronic textiles. Matter 4, 3725–3740 (2021).
Zhao, X. et al. Soft fibers with magnetoelasticity for wearable electronics. Nat. Commun. 12, 6755 (2021).
Xu, J. et al. A programmable magnetoelastic sensor array for self-powered human–machine interface. Appl. Phys. Rev. 9, 031404 (2022).
Zhou, Y. et al. A multimodal magnetoelastic artificial skin for underwater haptic sensing. Sci. Adv. 10, eadj8567 (2024).
Che, Z. et al. Speaking without vocal folds using a machine-learning-assisted wearable sensing-actuation system. Nat. Commun. 15, 1873 (2024).
Dobashi, Y. et al. Piezoionic mechanoreceptors: force-induced current generation in hydrogels. Science 376, 502–507 (2022).
Chen, K. & Ho, D. Piezoionics: mechanical‐to‐ionic transduction for sensing, biointerface, and energy harvesting. Aggregate 5, e425 (2023).
Zheng, Q. et al. Robust multilayered encapsulation for high-performance triboelectric nanogenerator in harsh environment. ACS Appl. Mater. Interfaces 8, 26697–26703 (2016).
Libanori, A. et al. Self-powered programming of fibroblasts into neurons via a scalable magnetoelastic generator array. Adv. Mater. 35, e2206933 (2023).
Zhao, X. et al. Giant magnetoelastic effect enabled stretchable sensor for self-powered biomonitoring. ACS Nano 16, 6013–6022 (2022).
Xu, J. et al. A textile magnetoelastic patch for self-powered personalized muscle physiotherapy. Matter 6, 2235–2247 (2023).
Jung, S. et al. Unraveling the missing link of bio‐electrical stimulation from body‐mediated energy transfer. Adv. Funct. Mater. 33, 2302465 (2023).
Yao, G. et al. A self-powered implantable and bioresorbable electrostimulation device for biofeedback bone fracture healing. Proc. Natl Acad. Sci. USA 118, e2100772118 (2021).
Wang, T. et al. Rehabilitation exercise-driven symbiotic electrical stimulation system accelerating bone regeneration. Sci. Adv. 10, eadi6799 (2024).
Yao, G. et al. Snowflake-inspired and blink-driven flexible piezoelectric contact lenses for effective corneal injury repair. Nat. Commun. 14, 3604 (2023).
Liu, S. et al. A neuroanatomical basis for electroacupuncture to drive the vagal–adrenal axis. Nature 598, 641–645 (2021).
Fox, D. The shock tactics set to shake up immunology. Nature 545, 20–22 (2017).
Yao, G. et al. Effective weight control via an implanted self-powered vagus nerve stimulation device. Nat. Commun. 9, 5349 (2018).
Zhang, Y. et al. Performance-enhanced flexible piezoelectric nanogenerator via layer-by-layer assembly for self-powered vagal neuromodulation. Nano Energy 89, 106319 (2021).
Won, S. M., Cai, L., Gutruf, P. & Rogers, J. A. Wireless and battery-free technologies for neuroengineering. Nat. Biomed. Eng. 7, 405–423 (2023).
Sun, Y. et al. Hybrid nanogenerator based closed-loop self-powered low-level vagus nerve stimulation system for atrial fibrillation treatment. Sci. Bull. 67, 1284–1294 (2022).
Oehrn, C. R. et al. Chronic adaptive deep brain stimulation versus conventional stimulation in Parkinson’s disease: a blinded randomized feasibility trial. Nat. Med. 30, 3345–3356 (2024).
Kang, W. et al. Closed-loop direct control of seizure focus in a rodent model of temporal lobe epilepsy via localized electric fields applied sequentially. Nat. Commun. 13, 7805 (2022).
Hollunder, B. et al. Mapping dysfunctional circuits in the frontal cortex using deep brain stimulation. Nat. Neurosci. 27, 573–586 (2024).
Photopoulos, J. Bioelectronic devices could treat autoimmune disease. Nature 595, S63 (2021).
Zhao, Q. et al. A multidimensional coding architecture of the vagal interoceptive system. Nature 603, 878–884 (2022).
Han, M. et al. Three-dimensional piezoelectric polymer microsystems for vibrational energy harvesting, robotic interfaces and biomedical implants. Nat. Electron. 2, 26–35 (2019).
Wang, F., Mai, Y.-W., Wang, D., Ding, R. & Shi, W. High quality barium titanate nanofibers for flexible piezoelectric device applications. Sens. Actuators A 233, 195–201 (2015).
Cordero, F. Quantitative evaluation of the piezoelectric response of unpoled ferroelectric ceramics from elastic and dielectric measurements: tetragonal BaTiO3. J. Appl. Phys. 123, 094103 (2018).
Maruyama, K., Kawakami, Y. & Narita, F. Young’s modulus and ferroelectric property of BaTiO3 films formed by aerosol deposition in consideration of residual stress and film thickness. Jpn. J. Appl. Phys. 61, 250204777 (2022).
Luo, Y. et al. Nanoshell tubes of ferroelectric lead zirconate titanate and barium titanate. Appl. Phys. Lett. 83, 440–442 (2003).
Jiang, Y., Man, G., Wang, X. & He, H. Identification of elastic-plastic and phase transition characteristics for relaxor ferroelectric PMN-PT anisotropic single crystals using nanoindentation technique. Philos. Mag. 98, 2595–2608 (2018).
Hwang, G. T. et al. Self-powered cardiac pacemaker enabled by flexible single crystalline PMN-PT piezoelectric energy harvester. Adv. Mater. 26, 4880–4887 (2014).
Shibata, K., Watanabe, K., Kuroda, T. & Osada, T. KNN lead-free piezoelectric films grown by sputtering. Appl. Phys. Lett. 121, 092901 (2022).
Zhao, M.-H., Wang, Z.-L. & Mao, S. X. Piezoelectric characterization of individual zinc oxide nanobelt probed by piezoresponse force microscope. Nano Lett. 4, 587–590 (2004).
Wu, H. S., Murti, B. T., Singh, J., Yang, P. K. & Tsai, M. L. Prospects of metal-free perovskites for piezoelectric applications. Adv. Sci. 9, e2104703 (2022).
Sekhar Muddam, R., Sinclair, J. & Krishnan Jagadamma, L. Piezoelectric charge coefficient of halide perovskites. Materials 17, 3083 (2024).
Guerin, S. et al. Control of piezoelectricity in amino acids by supramolecular packing. Nat. Mater. 17, 180–186 (2018).
Zhang, Z. et al. Active self-assembly of piezoelectric biomolecular films via synergistic nanoconfinement and in-situ poling. Nat. Commun. 14, 4094 (2023).
Shin, D.-M. et al. Bioinspired piezoelectric nanogenerators based on vertically aligned phage nanopillars. Energy Environ. Sci. 8, 3198–3203 (2015).
Kim, H. & Lee, S. W. Molecular mechanisms and enhancement of piezoelectricity in the M13 virus. Adv. Funct. Mater. 34, 2407462 (2024).
Choi, S. S. & Kim, K. J. Mechanical characterization of P2 bacteriophage by using Young’s modulus measurements. AIP Adv. 11, 015245 (2021).
Nguyen, V., Jenkins, K. & Yang, R. Epitaxial growth of vertically aligned piezoelectric diphenylalanine peptide microrods with uniform polarization. Nano Energy 17, 323–329 (2015).
Su, Y. et al. Electric field-assisted self-assembly of diphenylalanine peptides for high-performance energy conversion. ACS Mater. Lett. 5, 2317–2323 (2023).
Basavalingappa, V. et al. Diphenylalanine-derivative peptide assemblies with increased aromaticity exhibit metal-like rigidity and high piezoelectricity. ACS Nano 14, 7025–7037 (2020).
Fang, W. et al. Oriented strontium carbonate nanocrystals within collagen films for flexible piezoelectric sensors. Adv. Funct. Mater. 31, 2105806 (2021).
Denning, D. et al. Piezoelectric tensor of collagen fibrils determined at the nanoscale. ACS Biomater. Sci. Eng. 3, 929–935 (2017).
Andriotis, O. G., Nalbach, M. & Thurner, P. J. Mechanics of isolated individual collagen fibrils. Acta Biomater. 163, 35–49 (2023).
Romanyuk, K. et al. Piezoactive dense diphenylalanine thin films via solid-phase crystallization. Appl. Mater. Today 26, 101261 (2022).
Quesada Cabrera, R., Meersman, F., McMillan, P. F. & Dmitriev, V. Nanomechanical and structural properties of native cellulose under compressive stress. Biomacromolecules 12, 2178–2183 (2011).
Tanpichai, S. et al. Effective Young’s modulus of bacterial and microfibrillated cellulose fibrils in fibrous networks. Biomacromolecules 13, 1340–1349 (2012).
Zhai, L., Kim, H. C., Kim, J. W. & Kim, J. Alignment effect on the piezoelectric properties of ultrathin cellulose nanofiber films. ACS Appl. Bio Mater. 3, 4329–4334 (2020).
Chen, P. et al. Quantifying the contribution of the dispersion interaction and hydrogen bonding to the anisotropic elastic properties of chitin and chitosan. Biomacromolecules 23, 1633–1642 (2022).
de Marzo, G. et al. Sustainable, flexible, and biocompatible enhanced piezoelectric chitosan thin film for compliant piezosensors for human health. Adv. Electron. Mater. 9, 2200069 (2022).
Ribeiro, C., Sencadas, V., Correia, D. M. & Lanceros-Mendez, S. Piezoelectric polymers as biomaterials for tissue engineering applications. Colloids Surf. B 136, 46–55 (2015).
Chernozem, R. V. et al. Piezoelectric hybrid scaffolds mineralized with calcium carbonate for tissue engineering: analysis of local enzyme and small-molecule drug delivery, cell response and antibacterial performance. Mat. Sci. Eng. C 122, 111909 (2021).
Sultana, A. et al. Human skin interactive self-powered wearable piezoelectric bio-e-skin by electrospun poly-L-lactic acid nanofibers for non-invasive physiological signal monitoring. J. Mater. Chem. B 5, 7352–7359 (2017).
Pariy, I. O. et al. Hybrid biodegradable electrospun scaffolds based on poly(L-lactic acid) and reduced graphene oxide with improved piezoelectric response. Polym. J. 54, 1237–1252 (2022).
Jariyavidyanont, K. et al. Young’s modulus of the different crystalline phases of poly (L-lactic acid). J. Mech. Behav. Biomed. Mater. 137, 105546 (2023).
Kanik, M., Aktas, O., Sen, H. S., Durgun, E. & Bayindir, M. Spontaneous high piezoelectricity in poly(vinylidene fluoride) nanoribbons produced by iterative thermal size reduction technique. ACS Nano 8, 9311–9323 (2014).
Fang, F., Shan, S. C. & Yang, W. A multipeak phenomenon of magnetoelectric coupling in Terfenol-D/P(VDF-TrFE)/Terfenol-D laminates. J. Appl. Phys. 108, 104505 (2010).
Ikei, A., Wissman, J., Sampath, K., Yesner, G. & Qadri, S. N. Tunable in situ 3D-printed PVDF-TrFE piezoelectric arrays. Sensors 21, 5032 (2021).
Baji, A., Mai, Y.-W., Li, Q. & Liu, Y. Nanoscale investigation of ferroelectric properties in electrospun barium titanate/polyvinylidene fluoride composite fibers using piezoresponse force microscopy. Compos. Sci. Technol. 71, 1435–1440 (2011).
Kim, H. S. et al. Dominant role of Young’s modulus for electric power generation in PVDF–BaTiO3 composite-based piezoelectric nanogenerator. Nanomaterials 8, 777 (2018).
Huang, Z.-X. et al. Self-poled piezoelectric polymer composites via melt-state energy implantation. Nat. Commun. 15, 819 (2024).
Zhang, C., Wei, W., Sun, H. & Zhu, Q. Performance enhancements in poly(vinylidene fluoride)-based piezoelectric films prepared by the extrusion-casting process. J. Mater. Sci. Mater. Electron. 32, 21837–21847 (2021).
Li, J. et al. Multifunctional artificial artery from direct 3D printing with built‐In ferroelectricity and tissue‐matching modulus for real‐time sensing and occlusion monitoring. Adv. Funct. Mater. 30, 2002868 (2020).
Li, J. et al. Bulk ferroelectric metamaterial with enhanced piezoelectric and biomimetic mechanical properties from additive manufacturing. ACS Nano 15, 14903–14914 (2021).
Han, J. et al. Highly sensitive impact sensor based on PVDF-TrFE/Nano-ZnO composite thin film. Sensors 19, 830 (2019).
Chai, B. et al. Modulus-modulated all-organic core–shell nanofiber with remarkable piezoelectricity for energy harvesting and condition monitoring. Nano Lett. 23, 1810–1819 (2023).
Tang, T. et al. Stretchable polymer composites with ultrahigh piezoelectric performance. Natl Sci. Rev. 10, nwad177 (2023).
Li, H., Lee, H. B., Kang, J.-W. & Lim, S. Three-dimensional polymer-nanoparticle-liquid ternary composite for ultrahigh augmentation of piezoelectric nanogenerators. Nano Energy 113, 108576 (2023).
Zhang, Q., Zhu, J., Fei, X. & Zhu, M. A Janus nanofibrous scaffold integrated with exercise-driven electrical stimulation and nanotopological effect enabling the promotion of tendon-to-bone healing. Nano Today 55, 102208 (2024).
Shan, Y. et al. A biodegradable piezoelectric sensor for real‐time evaluation of the motor function recovery after nerve injury. Adv. Funct. Mater. 34, 2400295 (2024).
Chorsi, M. T. et al. Highly piezoelectric, biodegradable, and flexible amino acid nanofibers for medical applications. Sci. Adv. 9, eadg6075 (2023).
Xue, H. et al. Flexible, biodegradable ultrasonic wireless electrotherapy device based on highly self-aligned piezoelectric biofilms. Sci. Adv. 10, eadn0260 (2024).
Song, X. et al. Nanomedicine-enabled sonomechanical, sonopiezoelectric, sonodynamic, and sonothermal therapy. Adv. Mater. 35, e2212259 (2023).
Wei, Z. et al. Physical cue‐based strategies on peripheral nerve regeneration. Adv. Funct. Mater. 33, 2209658 (2022).
Zhou, L. et al. Wireless self-powered optogenetic system for long-term cardiac neuromodulation to improve post-MI cardiac remodeling and malignant arrhythmia. Adv. Sci. 10, e2205551 (2023).
Acknowledgements
Z.-Q.F., F.J. and T.L. received financial support from the National Natural Science Foundation of China (grants 82302406, 82472159, 52303186, 51773093 and 11204033). F.J. and T.L. acknowledge funding from China Postdoctoral Science Foundation (grants 2024T171167, 2023M731696, 2022TQ0158 and 2022M721616). F.J. and T.L. acknowledge funding from Jiangsu Funding Program for Excellent Postdoctoral Talent (grants 2023ZB539 and 2022ZB250). F.J. and Z.-Q.F received financial support from the Fundamental Research Funds for the Central Universities (grants 30923010307 and 30920041105).
Author information
Authors and Affiliations
Contributions
S.W. and F.J. researched data for the article and contributed substantially to discussions of its content. S.W., F.J., T.L., Z.W., L.Q., N.J. and Z.-Q.F. wrote the manuscript. All authors contributed to the review and/or editing of the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Electrical Engineering thanks Soyun Joo, who co-reviewed with Seungbum Hong, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jin, F., Li, T., Wei, Z. et al. A bright future for self-sustainable bioelectronics. Nat Rev Electr Eng 2, 338–349 (2025). https://doi.org/10.1038/s44287-025-00164-8
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44287-025-00164-8
This article is cited by
-
A Comprehensive Review on Conducting Polymers for Multifaceted Applications
Journal of Inorganic and Organometallic Polymers and Materials (2025)