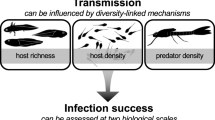
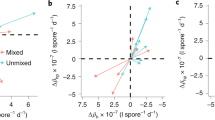
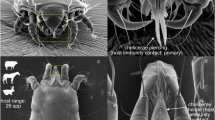
Abstract
Parasites are ubiquitous and frequently impose strong deleterious fitness effects on host individuals. These effects often manifest at the microevolutionary scale through host assortative mating and local adaptation. At the macroevolutionary scale, parasite-mediated effects can not only cause population divergence leading to speciation but also cause extirpation of host populations, leading to extinction. The balance between parasite-mediated effects on both speciation and extinction determines species diversification patterns. However, empirical tests of the hypothesis that parasitism contributes to macroevolutionary dynamics of host speciation and extinction are lacking. In this Perspective, we discuss how parasites can affect host macroevolution and outline an approach to determine whether parasitism and diversification are linked. We predict that parasitism, similar to other species interactions, shapes the process of diversification specifically in host species. Testing this hypothesis will require further empirical research to investigate the role of parasitism driving host diversification across the tree of life.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Schluter, D. Ecological character displacement in adaptive radiation. Am. Nat. 156, S4–S16 (2000).
Zeng, Y. & Wiens, J. J. Species interactions have predictable impacts on diversification. Ecol. Lett. 24, 239–248 (2021).
Aristide, L. & Morlon, H. Understanding the effect of competition during evolutionary radiations: an integrated model of phenotypic and species diversification. Ecol. Lett. 22, 2006–2017 (2019).
Nosil, P. Adaptive population divergence in cryptic color-pattern following a reduction in gene flow. Evolution 63, 1902–1912 (2009).
Thompson, J. N. The Geographic Mosaic of Coevolution (Univ. Chicago Press, 2005).
Fowler, M. S. Extinction cascades and the distribution of species interactions. Oikos 119, 864–873 (2010).
Karvonen, A. & Seehausen, O. The role of parasitism in adaptive radiations — when might parasites promote and when might they constrain ecological speciation? Int. J. Ecol. https://doi.org/10.1155/2012/280169 (2012).
Hasik, A. Z. & Siepielski, A. M. Parasitism shapes selection by drastically reducing host fitness and increasing host fitness variation. Biol. Lett. 18, 20220323 (2022).
Gobbin, T. P. et al. Temporally consistent species differences in parasite infection but no evidence for rapid parasite‐mediated speciation in Lake Victoria cichlid fish. J. Evol. Biol. 33, 556–575 (2020).
Gobbin, T. P., Vanhove, M. P. M., Veenstra, R., Maan, M. E. & Seehausen, O. Variation in parasite infection between replicates of speciation in Lake Victoria cichlid fish. Evolution 77, 1682–1690 (2023).
Raeymaekers, J. A. M. et al. Contrasting parasite communities among allopatric colour morphs of the Lake Tanganyika cichlid Tropheus. BMC Evol. Biol. 13, 1–16 (2013).
El Nagar, A. & MacColl, A. D. C. Parasites contribute to ecologically dependent postmating isolation in the adaptive radiation of three-spined stickleback. Proc. R. Soc. B 283, 20160691 (2016).
Malmstrøm, M. et al. Evolution of the immune system influences speciation rates in teleost fishes. Nat. Genet. 48, 1204–1210 (2016).
Morand, S. (macro-) Evolutionary ecology of parasite diversity: from determinants of parasite species richness to host diversification. Int. J. Parasitol. Parasites Wildl. 4, 80–87 (2015).
Poulin, R. Parasite biodiversity revisited: frontiers and constraints. Int. J. Parasitol. 44, 581–589 (2014).
Price, P. W. Evolutionary Biology of Parasites Vol. 15 (Princeton Univ. Press, 1980).
Ilvonen, J. J., Kaunisto, K. M. & Suhonen, J. Odonates, gregarines and water mites: why are the same host species infected by both parasites? Ecol. Entomol. 43, 591–600 (2018).
Gehman, A.-L. M. et al. Predators, environment and host characteristics influence the probability of infection by an invasive castrating parasite. Oecologia 183, 139–149 (2017).
Lyberger, K., Farner, J., Couper, L. & Mordecai, E. A. A mosquito parasite is locally adapted to its host but not temperature. Am. Nat. 204, 121–132 (2024).
Hamilton, W. D. & Zuk, M. Heritable true fitness and bright birds: a role for parasites? Science 218, 384–387 (1982).
Zahavi, A. Mate selection — a selection for a handicap. J. Theor. Biol. 53, 205–214 (1975).
Boots, M. & Sasaki, A. Parasite‐driven extinction in spatially explicit host–parasite systems. Am. Nat. 159, 706–713 (2002).
Hwang, T.-W. & Kuang, Y. Deterministic extinction effect of parasites on host populations. J. Math. Biol. 46, 17–30 (2003).
Auld, S. K. J. R., Tinkler, S. K. & Tinsley, M. C. Sex as a strategy against rapidly evolving parasites. Proc. R. Soc. B 283, 20162226 (2016).
Hamilton, W. D., Axelrod, R. & Tanese, R. Sexual reproduction as an adaptation to resist parasites (a review). Proc. Natl Acad. Sci. USA 87, 3566–3573 (1990).
Koch, H. R., Wagner, S. & Becks, L. Antagonistic species interaction drives selection for sex in a predator–prey system. J. Evol. Biol. 33, 1180–1191 (2020).
Haldane, J. B. S. Disease and evolution. La Ricerca Scientifica 19, 68–76 (1949).
Summers, K. et al. Parasitic exploitation as an engine of diversity. Biol. Rev. 78, 639–675 (2003).
Thompson, J. N. & Burdon, J. J. Gene-for-gene coevolution between plants and parasites. Nature 360, 121–125 (1992).
Brown, J. K. M. & Tellier, A. Plant-parasite coevolution: bridging the gap between genetics and ecology. Annu. Rev. Phytopathol. 49, 345–367 (2011).
Garamszegi, L. Z. & Nunn, C. L. Parasite‐mediated evolution of the functional part of the MHC in primates. J. Evol. Biol. 24, 184–195 (2011).
Klein, J., O’Huigin, C. & Deutsch, J. MHC polymorphism and parasites. Philos. Trans. R. Soc. B Biol. Sci. 346, 19940152 (1994).
Lheureux, F., Carreel, F., Jenny, C., Lockhart, B. & Iskra-Caruana, M. Identification of genetic markers linked to banana streak disease expression in inter-specific Musa hybrids. Theor. Appl. Genet. 106, 594–598 (2003).
Lockhart, B. E., Menke, J., Dahal, G. & Olszewski, N. E. Characterization and genomic analysis of tobacco vein clearing virus, a plant pararetrovirus that is transmitted vertically and related to sequences integrated in the host genome. J. Gen. Virol. 81, 1579–1585 (2000).
Ndowora, T. et al. Evidence that badnavirus infection in Musa can originate from integrated pararetroviral sequences. Virology 255, 214–220 (1999).
Roy, R. P. Semi-lethal hybrids in crosses of species and synthetic amphidipîoids of Triticum and Aegilops. Indian J. Genet. 15, 88–98 (1955).
Sears, E. R. Inviability of intergeneric hybrids involving Triticum monococcum and T. aegilopoides. Genetics 29, 113–127 (1944).
Bomblies, K. & Weigel, D. Hybrid necrosis: autoimmunity as a potential gene-flow barrier in plant species. Nat. Rev. Genet. 8, 382–393 (2007).
Engelstädter, J. & Fortun, N. Z. The dynamics of preferential host switching: host phylogeny as a key predictor of parasite distribution. Evolution 73, 1330–1340 (2019).
Betts, A., Gray, C., Zelek, M., MacLean, R. C. & King, K. C. High parasite diversity accelerates host adaptation and diversification. Science 360, 907–911 (2018).
Daszak, P. et al. Emerging infectious diseases and amphibian population declines.Emerg. Infect. Dis. 5, 735–748 (1999).
Valenzuela-Sánchez, A. et al. Cryptic disease-induced mortality may cause host extinction in an apparently stable host–parasite system. Proc. R. Soc. B Biol. Sci. 284, 20171176 (2017).
Castro, F. D. & Bolker, B. Mechanisms of disease-induced extinction. Ecol. Lett. 8, 117–126 (2005).
Safran, R. J., Scordato, E. S. C., Symes, L. B., Rodríguez, R. L. & Mendelson, T. C. Contributions of natural and sexual selection to the evolution of premating reproductive isolation: a research agenda. Trends Ecol. Evol. 28, 643–650 (2013).
Hooper, P. L. & Miller, G. F. Mutual mate choice can drive costly signaling even under perfect monogamy. Adapt. Behav. 16, 53–70 (2008).
Jiang, Y., Bolnick, D. I. & Kirkpatrick, M. Assortative mating in animals. Am. Nat. 181, E125–E138 (2013).
Hechtel, L. J., Johnson, C. L. & Juliano, S. A. Modification of antipredator behavior of Caecidotea intermedius by its parasite Acanthocephalus dirus. Ecology 74, 710–713 (1993).
Moore, J. Responses of an avian predator and its isopod prey to an acanthocephalan parasite. Ecology 64, 1000–1015 (1983).
Maan, M. E., Van Rooijen, A. M. C., Van Alphen, J. J. M. & Seehausen, O. Parasite-mediated sexual selection and species divergence in Lake Victoria cichlid fish. Biol. J. Linn. Soc. 94, 53–60 (2008).
Folstad, I., Hope, A. M., Karter, A. & Skorping, A. Sexually selected color in male sticklebacks: a signal of both parasite exposure and parasite resistance? Oikos 69, 511–515 (1994).
Selz, O. M., Pierotti, M. E. R., Maan, M. E., Schmid, C. & Seehausen, O. Female preference for male color is necessary and sufficient for assortative mating in 2 cichlid sister species. Behav. Ecol. 25, 612–626 (2014).
Milinski, M. & Bakker, T. C. M. Female sticklebacks use male coloration in mate choice and hence avoid parasitized males. Nature 344, 330–333 (1990).
Barber, I. & Scharsack, J. P. The three-spined stickleback-Schistocephalus solidus system: an experimental model for investigating host–parasite interactions in fish. Parasitology 137, 411–424 (2009).
Huchard, E., Baniel, A., Schliehe-Diecks, S. & Kappeler, P. M. MHC-disassortative mate choice and inbreeding avoidance in a solitary primate. Mol. Ecol. 22, 4071–4086 (2013).
Dandine-Roulland, C., Laurent, R., Dall’Ara, I., Toupance, B. & Chaix, R. Genomic evidence for MHC disassortative mating in humans. Proc. R. Soc. B 286, 20182664 (2019).
Penn, D. J., Damjanovich, K. & Potts, W. K. MHC heterozygosity confers a selective advantage against multiple-strain infections. Proc. Natl Acad. Sci. USA 99, 11260–11264 (2002).
Slade, J. W. G., Watson, M. J. & MacDougall-Shackleton, E. A. ‘Balancing’ balancing selection? Assortative mating at the major histocompatibility complex despite molecular signatures of balancing selection. Ecol. Evol. 9, 5146–5157 (2019).
Sepil, I. et al. No evidence for MHC class I‐based disassortative mating in a wild population of great tits. J. Evol. Biol. 28, 642–654 (2015).
Sin, Y. W. et al. MHC class II-assortative mate choice in European badgers (Meles meles). Mol. Biol. 24, 3138–3150 (2015).
Fraser, B. A. & Neff, B. D. Parasite mediated homogenizing selection at the MHC in guppies. Genetica 138, 273 (2010).
Eizaguirre, C., Lenz, T. L., Traulsen, A. & Milinski, M. Speciation accelerated and stabilized by pleiotropic major histocompatibility complex immunogenes. Ecol. Lett. 12, 5–12 (2009).
Preisser, W. Latitudinal gradients of parasite richness: a review and new insights from helminths of cricetid rodents. Ecography 42, 1315–1330 (2019).
Bolnick, D. I., Resetarits, E. J., Ballare, K., Stuart, Y. E. & Stutz, W. E. Scale‐dependent effects of host patch traits on species composition in a stickleback parasite metacommunity. Ecology 101, e03181 (2020).
Hasik, A. Z. & Siepielski, A. M. A role for the local environment in driving species‐specific parasitism in a multi‐host parasite system. Freshw. Biol. 67, 1571–1583 (2022).
Blasco-Costa, I., Rouco, C. & Poulin, R. Biogeography of parasitism in freshwater fish: spatial patterns in hot spots of infection. Ecography 38, 301–310 (2015).
Hasik, A. Z., Bried, J. T., Bolnick, D. I. & Siepielski, A. M. Is the local environment more important than within-host interactions in determining coinfection? J. Anim. Ecol. 93, 1541–1555 (2024).
Greischar, M. A. & Koskella, B. A synthesis of experimental work on parasite local adaptation. Ecol. Lett. 10, 418–434 (2007).
Céspedes, V., Stoks, R., Green, A. J. & Sánchez, M. I. Eco-immunology of native and invasive water bugs in response to water mite parasites: insights from phenoloxidase activity. Biol. Invasions 21, 2431–2445 (2019).
Mesquita, A. F. C. et al. Low resistance to chytridiomycosis in direct-developing amphibians. Sci. Rep. 7, 16605 (2017).
Hasik, A. Z., Tye, S. P., Ping, T. & Siepielski, A. M. A common measure of prey immune function is not constrained by the cascading effects of predators. Evol. Ecol. https://doi.org/10.1007/s10682-021-10124-x (2021).
Worthen, W. B. & Turner, L. H. The effects of odonate species abundance and diversity on parasitism by water mites (Arrenurus spp.): testing the dilution effect. Int. J. Odonatol. 18, 233–248 (2015).
Risely, A., Klaasen, M. & Hoye, B. J. Migratory animals feel the cost of getting sick: a meta-analysis across species. J. Anim. Ecol. 87, 301–314 (2017).
Phillips, K. P. et al. Immunogenetic novelty confers a selective advantage in host–pathogen coevolution. Proc. Natl Acad. Sci. USA 115, 1552–1557 (2018).
Bolnick, D. I. & Stutz, W. E. Frequency dependence limits divergent evolution by favouring rare immigrants over residents. Nature 546, 285–288 (2017).
Altizer, S., Nunn, C. L. & Lindenfors, P. Do threatened hosts have fewer parasites? A comparative study in primates. J. Anim. Ecol. 76, 304–314 (2007).
Bruns, E. L., Antonovics, J. & Hood, M. Is there a disease-free halo at species range limits? The codistribution of anther-smut disease and its host species. J. Ecol. 107, 1–11 (2019).
Tompkins, D. M., White, A. R. & Boots, M. Ecological replacement of native red squirrels by invasive greys driven by disease. Ecol. Lett. 6, 189–196 (2003).
Atkinson, M. S. & Savage, A. E. Invasive amphibians alter host–pathogen interactions with primarily negative outcomes for native species. Biol. Conserv. 286, 110310 (2023).
Wyatt, K. B. et al. Historical mammal extinction on Christmas Island (Indian Ocean) correlates with introduced infectious disease. PLoS ONE 3, e3602 (2008).
Ryan, M. J., Lips, K. R. & Eichholz, M. W. Decline and extirpation of an endangered Panamanian stream frog population (Craugastor punctariolus) due to an outbreak of chytridiomycosis. Biol. Conserv. 141, 1636–1647 (2008).
Hoyt, J. R. et al. Host persistence or extinction from emerging infectious disease: insights from white-nose syndrome in endemic and invading regions. Proc. R. Soc. B 283, 20152861 (2016).
Hasik, A. Z. et al. Resetting our expectations for parasites and their effects on species interactions: a meta-analysis. Ecol. Lett. 26, 184–199 (2023).
Reiczigel, J., Marozzi, M., Fábián, I. & Rózsa, L. Biostatistics for parasitologists — a primer to quantitative parasitology. Trends Parasitol. 35, 277–281 (2019).
Anderson, R. M. & May, R. M. The population dynamics of microparasites and their invertebrate hosts. Philos. Trans. R. Soc. B Biol. Sci. 291, 19810005 (1981).
Connell, J. H. Diversity and the coevolution of competitors, or the ghost of competition past. Oikos 35, 131–138 (1980).
Poulin, R. et al. Evolutionary signature of ancient parasite pressures, or the ghost of parasitism past. Front. Ecol. Evol. 8, 195 (2020).
Maddison, W. P. & FitzJohn, R. G. The unsolved challenge to phylogenetic correlation tests for categorical characters. Syst. Biol. 64, 127–136 (2015).
Beaulieu, J. M. & Donoghue, M. J. Fruit evolution and diversification in campanulid angiosperms. Evolution 67, 3132–3144 (2013).
Beaulieu, J. M. & O’Meara, B. C. Detecting hidden diversification shifts in models of trait-dependent speciation and extinction. Syst. Biol. 65, 583–601 (2016).
Rabosky, D. L. & Goldberg, E. E. FiSSE: a simple nonparametric test for the effects of a binary character on lineage diversification rates. Evolution 71, 1432–1442 (2017).
Rabosky, D. L. & Huang, H. A robust semi-parametric test for detecting trait-dependent diversification. Syst. Biol. 65, 181–193 (2015).
Caetano, D. S., O’Meara, B. C. & Beaulieu, J. M. Hidden state models improve state‐dependent diversification approaches, including biogeographical models. Evolution 72, 2308–2324 (2018).
Nakov, T., Beaulieu, J. M. & Alverson, A. J. Diatoms diversify and turn over faster in freshwater than marine environments. Evolution 73, 2497–2511 (2019).
Louca, S. & Pennell, M. W. Extant timetrees are consistent with a myriad of diversification histories. Nature 580, 502–505 (2020).
Heath, T. A., Huelsenbeck, J. P. & Stadler, T. The fossilized birth–death process for coherent calibration of divergence-time estimates. Proc. Natl Acad. Sci. USA 111, E2957–E2966 (2014).
Stadler, T., Gavryushkina, A., Warnock, R. C. M., Drummond, A. J. & Heath, T. A. The fossilized birth–death model for the analysis of stratigraphic range data under different speciation modes. J. Theor. Biol. 447, 41–55 (2018).
Leung, T. L. F. Fossils of parasites: what can the fossil record tell us about the evolution of parasitism? Biol. Rev. 92, 410–430 (2015).
Beaulieu, J. M. & O’Meara, B. C. Fossils do not substantially improve, and may even harm, estimates of diversification rate heterogeneity. Syst. Biol. 72, 50–61 (2023).
Uyeda, J. C., Hansen, T. F. & McPeek, M. A. The million-year wait for macroevolutionary bursts. Proc. Natl Acad. Sci. USA 108, 15908–15913 (2011).
Schluter, D. Variable success in linking micro- and macroevolution. Evol. J. Linn. Soc. 3, kzae016 (2024).
Tsuboi, M. et al. The paradox of predictability provides a bridge between micro- and macroevolution. J. Evol. Biol. 37, 1413–1432 (2024).
Ebert, D. & Fields, P. D. Host–parasite co-evolution and its genomic signature. Nat. Rev. Genet. 21, 754–768 (2020).
Hasik, Z. A., King, C. K. & Hawlena, H. Interspecific host competition and parasite virulence evolution. Biol. Lett. 19, 20220553 (2023).
Hite, J. L. & Cressler, C. E. Parasite-mediated anorexia and nutrition modulate virulence evolution. Integr. Comp. Biol. 59, 1264–1274 (2019).
Poulin, R. Chapter 1 — the many roads to parasitism: a tale of convergence. Adv. Parasitol. 74, 1–40 (2011).
Alizon, S., de Roode, J. C. & Michalakis, Y. Multiple infections and the evolution of virulence. Ecol. Lett. 16, 556–567 (2013).
Bordes, F. & Morand, S. The impact of multiple infections on wild animal hosts: a review. Infect. Ecol. Epidemiol. 1, 7346 (2011).
Clerc, M., Fenton, A., Babayan, S. A. & Pedersen, A. B. Parasitic nematodes simultaneously suppress and benefit from coccidian coinfection in their natural mouse host. Parasitology 146, 1096–1106 (2019).
Dallas, T. A., Laine, A.-L. & Ovaskainen, O. Detecting parasite associations within multi-species host and parasite communities. Proc. R. Soc. B 286, 20191109 (2019).
Brooks, D. R., Hoberg, E. P. & Boeger, W. A. In the eye of the cyclops: the classic case of cospeciation and why paradigms are important. Comp. Parasitol. 82, 1–8 (2015).
Pfenning-Butterworth, A. C., Davies, T. J. & Cressler, C. E. Identifying co-phylogenetic hotspots for zoonotic disease. Philos. Trans. R. Soc. B Biol. Sci. 376, 20200363 (2021).
Buckling, A. & Hodgson, D. J. Short-term rates of parasite evolution predict the evolution of host diversity. J. Evol. Biol. 20, 1682–1688 (2007).
Morran, L. T., Schmidt, O. G., Gelarden, I. A., Parrish, R. C. & Lively, C. M. Running with the Red Queen: host–parasite coevolution selects for biparental sex. Science 333, 216–218 (2011).
Marston, M. F. et al. Rapid diversification of coevolving marine Synechococcus and a virus. Proc. Natl Acad. Sci. USA 109, 4544–4549 (2012).
Nunn, C. L. et al. Parasites and the evolutionary diversification of primate clades. Am. Nat. 10.0003-0147/2004/1640S5-40138$15.00 (2004).
Mollentze, N. & Streicker, D. G. Viral zoonotic risk is homogenous among taxonomic orders of mammalian and avian reservoir hosts. Proc. Natl Acad. Sci. USA 117, 9423–9430 (2020).
Kamiya, T., O’Dwyer, K., Nakagawa, S. & Poulin, R. Host diversity drives parasite diversity: meta-analytical insights into patterns and causal mechanisms. Ecography 37, 689–697 (2014).
Albery, G. F. et al. Divergent age-related changes in parasite infection occur independently of behaviour and demography in a wild ungulate. Philos. Trans. R. Soc. B https://doi.org/10.1098/rstb.2023.0508 (2024).
Albery, G. F. et al. Seasonality of helminth infection in wild red deer varies between individuals and between parasite taxa. Parasitology 145, 1410–1420 (2018).
Hite, J., Pfenning, A. C. & Cressler, C. E. Starving the enemy? Feeding behavior shapes host–parasite interactions. Trends Ecol. Evol. 35, 68–80 (2020).
Revell, L. J. & Harmon, L. J. Phylogenetic Comparative Methods in R (Princeton Univ. Press, 2022).
Weber, M. G. & Agrawal, A. A. Phylogeny, ecology, and the coupling of comparative and experimental approaches. Trends Ecol. Evol. 27, 394–403 (2012).
Osigus, H.-J., Rolfes, S., Herzog, R., Kamm, K. & Schierwater, B. Polyplacotoma mediterranea is a new ramified placozoan species. Curr. Biol. 29, R148–R149 (2019).
Zhang, Z.-Q. Phylum arthropoda. Zootaxa 3703, 1–82 (2013).
Howard, V., Landis, J. B., Beaulieu, J. M. & Cellinese, N. Geophytism in monocots leads to higher rates of diversification. Phytologist 225, 1023–1032 (2020).
Pausas, J. G., Lamont, B. B., Paula, S., Appezzato-da-Glória, B. & Fidelis, A. Unearthing belowground bud banks in fire-prone ecosystems. N. Phytol. 217, 1435–1448 (2018).
Acknowledgements
The authors acknowledge support from NSF (DEB 2306183 awarded to A.M.S. and DEB 1916558 awarded to J.M.B.), Arkansas Biosciences Institute (A.M.S.), Academy of Finland (J.S.), University of Turku Graduate School (J.J.I.) and Emil Aaltonen Foundation (J.J.I.).
Author information
Authors and Affiliations
Contributions
All authors contributed substantially to discussion of the content. A.Z.H. wrote the article. All authors reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Biodiversity thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Abundance
-
The mean number of parasite individuals per host individual, considering both infected and uninfected hosts.
- Coevolutionary arms-race
-
Evolutionary scenario in which a competing set of co-evolving genotypes or phenotypes develop increasingly escalating adaptations and counter-adaptations.
- Diversification
-
The process by which the diversity of a group of organisms increases over time.
- Ecological speciation
-
The process by which new species form owing to the influence of divergent selection experienced in differing ecological environments.
- Extinction
-
The termination of a taxon when the last member dies.
- Extirpation
-
Complete elimination of a species from a specific area.
- Gene-for-gene models
-
Infection model whereby a parasite has a ‘universal virulence’ allowing it to infect all host genotypes.
- Hybrid necrosis
-
A type of postzygotic incompatibility seen across many species in which hybrid offspring are rarely able to reach their reproductive stage.
- Infection profile
-
Both the number of individual parasites and parasite species infecting a given host.
- Intensity
-
The mean number of parasite individuals per infected host individual.
- Local adaptation
-
Process by which an individual (or group of individuals) performs better in their local habitat than they would in another locality within their species’ geographical range.
- Matching allele infection models
-
Infection model whereby a parasite’s genotype must exactly match a host’s genotype to successfully infect the host.
- Parasites
-
Organisms that have evolved to acquire resources from a host organism at the expense of the host.
- Prevalence
-
The proportion of individuals within a given host population that are infected with a parasite.
- Red Queen dynamics
-
Evolutionary scenario in which species must adapt to and overcome the fitness costs imposed on them by antagonistic species that are also evolving.
- Reproductive isolation
-
Processes preventing members of different populations from successfully mating or producing viable offspring.
- Stockholm Paradigm
-
An evolutionary framework explaining macroevolutionary mechanisms that incorporate evolutionary and ecological processes.
- Tolerance
-
The strategy whereby hosts limit the harm suffered from increasing parasite loads.
- Turnover
-
The process by which a clade changes as lineages appear and disappear over time.
- Virulence
-
The harm to hosts caused by parasite infection.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hasik, A.Z., Ilvonen, J.J., Gobbin, T.P. et al. Parasitism as a driver of host diversification. Nat. Rev. Biodivers. 1, 401–410 (2025). https://doi.org/10.1038/s44358-025-00045-w
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44358-025-00045-w
This article is cited by
-
Parasite-mediated inbreeding depression in wild red deer
Heredity (2025)