Abstract
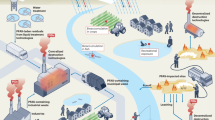
Thermal technologies, including incineration, pyrolysis and thermal desorption, are full-scale options for removing per- and polyfluoroalkyl substances (PFAS) from contaminated media. However, these thermal treatments generate products of incomplete destruction (PIDs), and complete PFAS mineralization is rarely achieved below 950 °C. In this Review, we examine the thermal degradation and mineralization pathways of PFAS and the PID-formation mechanisms, and highlight innovative strategies to enhance PFAS mineralization at reduced temperatures. PFAS-removal efficiencies are excellent (over 90%) across thermal technologies provided that high temperatures (above 700 °C) are used; however, mineralization efficiencies are generally less than 40% at temperatures below 700 °C and accompanied by the formation of PIDs, including perfluorocarbon greenhouse gases. Thermal phase transitions of PFAS (solid or sorbed to molten to vapour states) typically precede PFAS decomposition. Vapour containment is therefore essential to minimize fugitive emissions. The use of additives (activated carbon, alkali metals, alkaline-earth metals and platinum-group metals) can substantially minimize PID formation and improve mineralization (to more than 95%) at moderate temperatures (200–500 °C). However, additive-enhanced approaches are at varying stages of readiness, and further validation and process optimization are needed prior to large-scale implementation.
Key points
-
The thermal degradation of per- and polyfluoroalkyl substances (PFAS) is linked to their phase transitions, which determine whether degradation occurs in the condensed or vapour phase. Understanding these phase-dependent PFAS degradation processes and correspondingly incorporating melting, volatilization and gas-phase reactions into reactor design will be necessary to mitigate fugitive PFAS emissions.
-
The efficiency of conventional thermal technologies for PFAS degradation varies with temperature, oxygen availability and the material being treated. The major drawbacks of these systems include incomplete PFAS mineralization, the formation of products of incomplete destruction (PIDs) and fugitive PFAS emissions.
-
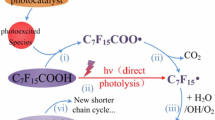
PIDs, which include fluorinated greenhouse gases, result from chain-scission and radical-mediated pathways. The apparent PFAS degradation estimated by the mass loss often overestimates the true extent of mineralization, because PFAS can transform into PIDs instead of into final inorganic products.
-
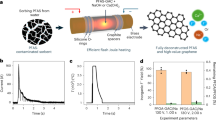
Granular activated carbon increases the mineralization of perfluorooctanoic acid and its homologues by as much as tenfold at 400 °C or less by concentrating PFAS vapours on its surface, facilitating C–F bond cleavage, and suppressing PID formation. However, its performance is moderate for high-boiling-point PFAS (such as perfluorooctane sulfonic acid) and needs to be further improved.
-
The addition of alkali and alkaline-earth metal additives converts fluorine radicals and hydrogen fluoride (fluorane, HF) into stable inorganic calcium fluoride (CaF2), resulting in PFAS mineralization of more than 95% at 500 °C while quenching HF and volatile PID emissions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data generated and/or analysed during this study are included in this article and its Supplementary Information.
References
Wang, Z., DeWitt, J. C., Higgins, C. P. & Cousins, I. T. A never-ending story of per- and polyfluoroalkyl substances (PFASs)? Environ. Sci. Technol. 51, 2508–2518 (2017).
Buck, R. C. et al. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr. Env. Assess. Manag. 7, 513–541 (2011).
Prevedouros, K., Cousins, I. T., Buck, R. C. & Korzeniowski, S. H. Sources, fate and transport of perfluorocarboxylates. Environ. Sci. Technol. 40, 32–44 (2006).
Barzen-Hanson, K. A. et al. Discovery of 40 classes of per- and polyfluoroalkyl substances in historical aqueous film-forming foams (AFFFs) and AFFF-impacted groundwater. Environ. Sci. Technol. 51, 2047–2057 (2017).
Lang, J. R., Allred, B. M., Peaslee, G. F., Field, J. A. & Barlaz, M. A. Release of per- and polyfluoroalkyl substances (PFASs) from carpet and clothing in model anaerobic landfill reactors. Environ. Sci. Technol. 50, 5024–5032 (2016).
Whitehead, H. D. et al. Fluorinated compounds in North American cosmetics. Environ. Sci. Tech. Lett. 8, 538–544 (2021).
Takagi, S. et al. Fate of perfluorooctanesulfonate and perfluorooctanoate in drinking water treatment processes. Water Res. 45, 3925–3932 (2011).
Eschauzier, C., Beerendonk, E., Scholte-Veenendaal, P. & de Voogt, P. Impact of treatment processes on the removal of perfluoroalkyl acids from the drinking water production chain. Environ. Sci. Technol. 46, 1708–1715 (2012).
Mejia Avendano, S., Zhong, G. & Liu, J. Comment on “Biodegradation of perfluorooctanesulfonate (PFOS) as an emerging contaminant”. Chemosphere 138, 1037–1038 (2015).
Yu, J., Hu, J. Y., Tanaka, S. & Fujii, S. Perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) in sewage treatment plants. Water Res. 43, 2399–2408 (2009).
Giesy, J. P. & Kannan, K. Global distribution of perfluorooctane sulfonate in wildlife. Environ. Sci. Technol. 35, 1339–1342 (2001).
Greaves, A. K., Letcher, R. J., Sonne, C., Dietz, R. & Born, E. W. Tissue-specific concentrations and patterns of perfluoroalkyl carboxylates and sulfonates in East Greenland polar bears. Environ. Sci. Technol. 46, 11575–11583 (2012).
Boisvert, G., Sonne, C., Riget, F. F., Dietz, R. & Letcher, R. J. Bioaccumulation and biomagnification of perfluoroalkyl acids and precursors in East Greenland polar bears and their ringed seal prey. Environ. Pollut. 252, 1335–1343 (2019).
Conder, J. M., Hoke, R. A., De Wolf, W., Russell, M. H. & Buck, R. C. Are PFCAs bioaccumulative? A critical review and comparison with regulatory criteria and persistent lipophilic compounds. Environ. Sci. Technol. 42, 995–1003 (2008).
Song, X. W., Vestergren, R., Shi, Y. L. & Cai, Y. Q. A matrix-correction approach to estimate the bioaccumulation potential of emerging PFASs. Environ. Sci. Technol. 54, 1005–1013 (2020).
Langberg, H. A. et al. Bioaccumulation of fluorotelomer sulfonates and perfluoroalkyl acids in marine organisms living in aqueous film-forming foam impacted waters. Environ. Sci. Technol. 53, 10951–10960 (2019).
Gronnestad, R. et al. Levels, patterns, and biomagnification potential of perfluoroalkyl substances in a terrestrial food chain in a Nordic skiing area. Environ. Sci. Technol. 53, 13390–13397 (2019).
Fourth National Report on Human Exposure to Environmental Chemicals. Updated Tables, March 2021: Volume Three: Analysis of Pooled Serum Samples for Select Chemicals, NHANES 2005–2016. National Center for Environmental Health https://stacks.cdc.gov/view/cdc/105344 (2014).
Lau, C. et al. Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol. Sci. 99, 366–394 (2007).
Grandjean, P. et al. Serum vaccine antibody concentrations in children exposed to perfluorinated compounds. JAMA 307, 391–397 (2012).
Beans, C. How “forever chemicals” might impair the immune system. Proc. Natl Acad. Sci. USA 118, e2105018118 (2021).
Fenton, S. E. et al. Per- and polyfluoroalkyl substance toxicity and human health review: current state of knowledge and strategies for informing future research. Environ. Toxicol. Chem. 40, 606–630 (2021).
Hoffman, K., Webster, T. F., Weisskopf, M. G., Weinberg, J. & Vieira, V. M. Exposure to polyfluoroalkyl chemicals and attention deficit/hyperactivity disorder in U.S. children 12–15 years of age. Environ. Health Persp. 118, 1762–1767 (2010).
Per- and polyfluoroalkyl substances (PFAS). Final PFAS National Primary Drinking Water Regulation. United States Environmental Protection Agency https://www.epa.gov/sdwa/and-polyfluoroalkyl-substances-pfas (2025).
Designation of PFOA and PFOS as hazardous substances under CERCLA Release Reporting Requirements Factsheet. United States Environmental Protection Agency https://www.epa.gov/epcra/designation-pfoa-and-pfos-hazardous-substances-under-cercla-release-reporting-requirements (2024).
Xiao, F. et al. Cross-national challenges and strategies for PFAS regulatory compliance in water infrastructure. Nat. Water 1, 1004–1015 (2023).
Per- and polyfluoroalkyl substances (PFAS). European Food Safety Authority https://www.efsa.europa.eu/en/topics/per-and-polyfluoroalkyl-substances-pfas (2024).
Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the quality of water intended for human consumption. Access to European Union law https://eur-lex.europa.eu/eli/dir/2020/2184/oj (2020).
Guidelines for Canadian drinking water quality: guideline technical document — perfluorooctanoic acid (PFOA) Government of Canada https://publications.aws.tpsgc-pwgsc.cloud-nuage.canada.ca/site/eng/9.858757/publication.html (2018).
Guidelines for Canadian drinking water quality: guideline technical document — perfluorooctane sulfonate (PFOS) Government of Canada https://open.canada.ca/data/en/info/e94c83cb-e311-4d31-a0aa-0c4611ba43e8 (2022).
Standards for drinking water quality (GB 5749-2022). Ministry of Health of the PR China https://openstd.samr.gov.cn/bzgk/gb/newGbInfo?hcno=99E9C17E3547A3C0CE2FD1FFD9F2F7BE [in Chinese] (2022).
Ateia, M. & Scheringer, M. From “forever chemicals” to fluorine-free alternatives. Science 385, 256–258 (2024).
PFAS national primary drinking water regulation rulemaking. United States Environmental Protection Agency https://www.epa.gov/system/files/documents/2023-03/Pre-Publication%20Federal%20Register%20Notice_PFAS%20NPDWR_NPRM_Final_3.13.23.pdf (2023).
Urtiaga, A., Gomez-Lavin, S. & Soriano, A. Electrochemical treatment of municipal landfill leachates and implications for poly-and perfluoroalkyl substances (PFAS) removal. J. Environ. Chem. Eng. 10, 107900 (2022).
Schaefer, C. E. et al. Electrochemical treatment of poly- and perfluoroalkyl substances in brines. Environ. Sci. Water Res. Technol. 6, 2704–2712 (2020).
Schaefer, C. E. et al. Electrochemical treatment of perfluorooctanoic acid and perfluorooctane sulfonate: insights into mechanisms and application to groundwater treatment. Chem. Eng. J. 317, 424–432 (2017).
Shende, T., Andaluri, G. & Suri, R. Chain-length dependent ultrasonic degradation of perfluoroalkyl substances. Chem. Eng. J. Adv. 15, 100509 (2023).
James Wood, R. et al. Ultrasonic degradation of perfluorooctane sulfonic acid (PFOS) correlated with sonochemical and sonoluminescence characterisation. Ultrason. Sonochem. 68, 105196 (2020).
Liu, F. Q., Guan, X. H. & Xiao, F. Photodegradation of per- and polyfluoroalkyl substances in water: a review of fundamentals and applications. J. Hazard. Mater. 439, 129580 (2022).
Qanbarzadeh, M. et al. An ultraviolet/boron nitride photocatalytic process efficiently degrades poly-/perfluoroalkyl substances in complex water matrices. Environ. Sci. Tech. Lett. 10, 705–710 (2023).
Amador, C. K., Vyas, S. & Strathmann, T. J. Kinetic model for predicting perfluoroalkyl acid degradation during UV-sulfite treatment. Environ. Sci. Technol. 58, 6425–6434 (2024).
Kewalramani, J. A., Wang, B., Marsh, R. W., Meegoda, J. N. & Rodriguez Freire, L. Coupled high and low-frequency ultrasound remediation of PFAS-contaminated soils. Ultrason. Sonochem. 88, 106063 (2022).
Kang, Y., Birch, Q. T., Nadagouda, M. N. & Dionysiou, D. D. Advanced destruction technologies for PFAS in soils: progress and challenges. Curr. Opin. Environ. Sci. Health 33, 100459 (2023).
Srivastava, P. & Macdonald, B. PFAS in biosolids: insights into current and future challenges. J. Hazard. Mater. Lett. 6, 100163 (2025).
Alinezhad, A. et al. An investigation of thermal air degradation and pyrolysis of per- and polyfluoroalkyl substances and aqueous film-forming foams in soil. ACS ES&T Eng. 2, 198–209 (2022).
Thoma, E. D. et al. Pyrolysis processing of PFAS-impacted biosolids, a pilot study. J. Air Waste Manag. Assoc. 72, 309–318 (2022).
Fournie, T., Rashwan, T. L., Switzer, C. & Gerhard, J. I. Smouldering to treat PFAS in sewage sludge. Waste Manag. 164, 219–227 (2023).
Duchesne, A. L. et al. Remediation of PFAS-contaminated soil and granular activated carbon by smoldering combustion. Environ. Sci. Technol. 54, 12631–12640 (2020).
Crownover, E., Oberle, D., Kluger, M. & Heron, G. Perfluoroalkyl and polyfluoroalkyl substances thermal desorption evaluation. Remediation 29, 77–81 (2019).
DiStefano, R., Feliciano, T., Mimna, R. A., Redding, A. M. & Matthis, J. Thermal destruction of PFAS during full-scale reactivation of PFAS-laden granular activated carbon. Remediation 32, 231–238 (2023).
Weber, N. H. et al. Kinetics of decomposition of PFOS relevant to thermal desorption remediation of soils. Ind. Eng. Chem. Res. 60, 9080–9087 (2021).
Kundu, S. et al. Removal of PFASs from biosolids using a semi-pilot scale pyrolysis reactor and the application of biosolids derived biochar for the removal of PFASs from contaminated water. Environ. Sci. Water Res. Technol. 7, 638–649 (2021).
Pinkard, B. R., Shetty, S., Stritzinger, D., Bellona, C. & Novosselov, I. V. Destruction of perfluorooctanesulfonate (PFOS) in a batch supercritical water oxidation reactor. Chemosphere 279, 130834 (2021).
Chiang, S. D. et al. Supercritical water oxidation for the destruction of spent media wastes generated from PFAS treatment. J. Hazard. Mater. 460, 132264 (2023).
Yang, M. et al. Experimental and theoretical insight of perfluorooctanoic acid destruction by alkaline hydrothermal treatment enhanced with zero-valent iron in biochar. ACS ES&T Water 3, 1286–1293 (2023).
Singh, R. K. et al. Rapid removal of poly- and perfluorinated compounds from investigation-derived waste (IDW) in a pilot-scale plasma reactor. Environ. Sci. Technol. 53, 11375–11382 (2019).
Tenorio, R. et al. Destruction of per- and polyfluoroalkyl substances (PFASs) in aqueous film-forming foam (AFFF) with UV-sulfite photoreductive treatment. Environ. Sci. Technol. 54, 6957–6967 (2020).
Li, J., Pinkard, B. R., Wang, S. & Novosselov, I. V. Review: hydrothermal treatment of per- and polyfluoroalkyl substances (PFAS). Chemosphere 307, 135888 (2022).
Xiao, F. et al. Thermal stability and decomposition of perfluoroalkyl substances on spent granular activated carbon. Environ. Sci. Tech. Lett. 7, 343–350 (2020).
West, C. P., Brown, H. M. & Fedick, P. W. Molecular characterization of the thermal degradation of per- and polyfluoroalkyl substances in aqueous film-forming foams via temperature-programmed thermal desorption-pyrolysis-direct analysis in real time-mass spectrometry. Environ. Sci. Tech. Lett. 10, 308–315 (2023).
Baghirzade, B. S. et al. Thermal regeneration of spent granular activated carbon presents an opportunity to break the forever PFAS cycle. Environ. Sci. Technol. 55, 5608–5619 (2021).
Xiao, F., Sasi, P. C., Alinezhad, A., Sun, R. & Abdulmalik Ali, M. Thermal phase transition and rapid degradation of forever chemicals (PFAS) in spent media using induction heating. ACS ES&T Eng. 3, 1370–1380 (2023).
Ateia, M., Maroli, A., Tharayil, N. & Karanfil, T. The overlooked short- and ultrashort-chain poly- and perfluorinated substances: a review. Chemosphere 220, 866–882 (2019).
Blotevogel, J., Giraud, R. J. & Rappé, A. K. Incinerability of PFOA and HFPO-DA: mechanisms, kinetics, and thermal stability ranking. Chem. Eng. J. 457, 141235 (2023).
Shields, E. P. et al. Pilot-scale thermal destruction of per- and polyfluoroalkyl substances in a legacy aqueous film forming foam. ACS ES&T Eng. 3, 1308–1317 (2023).
Gehrmann, H. J. et al. Mineralization of fluoropolymers from combustion in a pilot plant under representative European municipal and hazardous waste combustor conditions. Chemosphere 365, 143403 (2024).
Winchell, L. J. et al. Fate of perfluoroalkyl and polyfluoroalkyl substances (PFAS) through two full-scale wastewater sludge incinerators. Water Environ. Res. 96, e11009 (2024).
Strandberg, J. et al. PFAS in waste residuals from Swedish incineration plants: a systematic investigation. IVL https://www.ivl.se/download/18.556fc7e17c75c849331b76d/1636533451380/B2422%20PFAS%20from%20Swedish%20Waste%20Incineration%20Plants.pdf (2021).
Wohlin, D. Analysis of PFAS in ash from incineration facilities from Sweden. Orebro Universitet https://www.diva-portal.org/smash/get/diva2:1473805/FULLTEXT01.pdf (2021).
Liu, S. et al. Perfluoroalkyl substances (PFASs) in leachate, fly ash, and bottom ash from waste incineration plants: implications for the environmental release of PFAS. Sci. Total Environ. 795, 148468 (2021).
Altarawneh, M., Almatarneh, M. H. & Dlugogorski, B. Z. Thermal decomposition of perfluorinated carboxylic acids: kinetic model and theoretical requirements for PFAS incineration. Chemosphere 286, 131685 (2022).
Seay, B. A. et al. Per- and polyfluoroalkyl substances fate and transport at a wastewater treatment plant with a collocated sewage sludge incinerator. Sci. Total Environ. 874, 162357 (2023).
Weber, N. H. et al. Formation of products of incomplete destruction (PID) from the thermal oxidative decomposition of perfluorooctanoic acid (PFOA): measurement, modeling, and reaction pathways. J. Phys. Chem. A 128, 5362–5373 (2024).
Weber, N. H. et al. Modeling and experimental study on the thermal decomposition of perfluorooctanesulfonic acid (PFOS) in an α-alumina reactor. Ind. Eng. Chem. Res. 61, 5453–5463 (2022).
Weber, N. H. et al. Thermal decomposition of PFOA: influence of reactor and reaction conditions on product formation. Chem. Eng. Sci. 278, 118924 (2023).
Yao, B. et al. The first quantitative investigation of compounds generated from PFAS, PFAS-containing aqueous film-forming foams and commercial fluorosurfactants in pyrolytic processes. J. Hazard. Mater. 436, 129313 (2022).
Krusic, P. J. & Roe, D. C. Gas-phase NMR technique for studying the thermolysis of materials: thermal decomposition of ammonium perfluorooctanoate. Anal. Chem. 76, 3800–3803 (2004).
Alinezhad, A. et al. Mechanistic investigations of thermal decomposition of perfluoroalkyl ether carboxylic acids and short-chain perfluoroalkyl carboxylic acids. Environ. Sci. Technol. 57, 8796–8807 (2023).
Sasi, P. C. et al. Effect of granular activated carbon and other porous materials on thermal decomposition of per- and polyfluoroalkyl substances: mechanisms and implications for water purification. Water Res. 200, 117271 (2021).
Xiao, F. et al. Thermal decomposition of anionic, awitterionic, and cationic polyfluoroalkyl substances in aqueous film-forming foams. Environ. Sci. Technol. 55, 9885–9894 (2021).
Wang, J., Song, M., Abusallout, I. & Hanigan, D. Thermal decomposition of two gaseous perfluorocarboxylic acids: products and mechanisms. Environ. Sci. Technol. 57, 6179–6187 (2023).
Adi, M. A. & Altarawneh, M. Formation of perfluorocarboxylic acids (PFCAs) from thermolysis of Teflon model compound. Environ. Sci. Pollut. Res. Int. 30, 21360–21367 (2023).
AR5 synthesis report: climate change 2014. Intergovernmental Panel on Climate Change https://www.ipcc.ch/report/ar5/syr/ (2014).
Blotevogel, J., Joyce, J., Hill, O. & Rappe, A. Headgroup dependence and kinetic bottlenecks of gas-phase thermal pfas destruction. ACS ES&T Eng. 5, 910–921 (2025).
Troxler, W. L. et al. PFAS destruction by a hazardous waste incinerator: testing results. United States Environmental Protection Agency https://cfpub.epa.gov/si/si_public_record_Report.cfm?dirEntryId=367138&Lab=CEMM (2025).
Dolatabad, A. A. et al. Thermal decomposition of fluoropolymers: stability, decomposition products, and possible PFAS release. J. Hazard. Mater. 496, 139322 (2025).
Verma, S., Lee, T., Sahle-Demessie, E., Ateia, M. & Nadagouda, M. N. Recent advances on PFAS degradation via thermal and nonthermal methods. Chem. Eng. J. Adv. 13, 100421 (2023).
Weitz, K. et al. Review of per- and poly-fluoroalkyl treatment in combustion-based thermal waste systems in the United States. Sci. Total Environ. 932, 172658 (2024).
Longendyke, G. K., Katel, S. & Wang, Y. X. PFAS fate and destruction mechanisms during thermal treatment: a comprehensive review. Environ. Sci. Proc. Imp. 24, 196–208 (2022).
Wang, J. et al. Critical review of thermal decomposition of per- and polyfluoroalkyl substances: mechanisms and implications for thermal treatment processes. Environ. Sci. Technol. 56, 5355–5370 (2022).
Dolatabad, A. A., Mai, J., Zhang, X. & XIao, F. Fluorine mass flow in long-chain perfluoroalkyl carboxylic acids during thermal regeneration of granular activated carbon. J. Water Process. Eng. 70, 106990 (2025).
Sun, R. Z. et al. New insights into thermal degradation products of long-chain per- and polyfluoroalkyl substances (PFAS) and their mineralization enhancement using additives. Environ. Sci. Technol. 58, 22417–22430 (2024).
Ye, Y. et al. Release of volatile per- and polyfluoroalkyl substances from plant fiber-based food packaging and municipal solid waste to gas under simulated landfill conditions. Environ. Sci. Technol. 58, 21295–21304 (2024).
Lin, A. M. et al. Landfill gas: a major pathway for neutral per- and polyfluoroalkyl substance (PFAS) release. Environ. Sci. Technol. Lett. 11, 730–737 (2024).
Rahman, A. et al. Understanding dynamics of PFAS in biosolids processed through composting, thermal drying and high temperature pyrolysis. J. Water Process. Eng. 72, 107508 (2025).
Soker, O., Hao, S. L., Trewyn, B. G., Higgins, C. P. & Strathmann, T. J. Application of hydrothermal alkaline treatment to spent granular activated carbon: destruction of adsorbed PFASs and adsorbent regeneration. Environ. Sci. Technol. Lett. 10, 425–430 (2023).
Hao, S., Choi, Y. J., Deeb, R. A., Strathmann, T. J. & Higgins, C. P. Application of hydrothermal alkaline treatment for destruction of per- and polyfluoroalkyl substances in contaminated groundwater and soil. Environ. Sci. Technol. 56, 6647–6657 (2022).
Zhang, W. L. & Liang, Y. N. Effects of hydrothermal treatments on destruction of per- and polyfluoroalkyl substances in sewage sludge. Environ. Pollut. 285, 117276 (2021).
Li, J. N., Austin, C., Moore, S., Pinkard, B. R. & Novosselov, I. V. PFOS destruction in a continuous supercritical water oxidation reactor. Chem. Eng. J. 451, 139063 (2023).
Krause, M. J. et al. Supercritical water oxidation as an innovative technology for PFAS destruction. J. Environ. Eng. 148, 1–8 (2022).
Divine, C., March, L., Kalra, S. S. & Hurst, J. Sonolysis and super critical water oxidation (SCWO): development maturity and potential for destroying PFAS. Ground Water Monit. Remed. 43, 18–33 (2023).
Murakami, T., Saito, N., Matsukami, H., Takaoka, M. & Fujimori, T. Destruction of perfluorooctanoic acid (PFOA) and perfluorooctadecanoic acid (PFOcDA) by incineration: analysis of the by-products and their characteristics. Chemosphere 373, 144165 (2025).
Khan, M. Y., So, S. & da Silva, G. Decomposition kinetics of perfluorinated sulfonic acids. Chemosphere 238, 124615 (2020).
Other Test Method 50 (OTM-50): Sampling and analysis of volatile fluorinated compounds from stationary sources using passivated stainless-steel canisters. United States Environmental Protection Agency https://www.epa.gov/system/files/documents/2024-01/otm-50-release-1_0.pdf (2024).
Bjorklund, S., Weidemann, E. & Jansson, S. Emission of per- and polyfluoroalkyl substances from a waste-to-energy plant horizontal line occurrence in ashes, treated process water, and first observation in flue gas. Environ. Sci. Technol. 57, 10089–10095 (2023).
Toraman, H. E. EGEE 439: Alternative fuels from biomass sources: Biomass pyrolysis. Penn State College of earth and Mineral Sciences https://www.e-education.psu.edu/egee439/node/537 (2024).
Sormo, E. et al. Waste timber pyrolysis in a medium-scale unit: emission budgets and biochar quality. Sci. Total Environ. 718, 137335 (2020).
Chen, G. Y. et al. Products distribution and pollutants releasing characteristics during pyrolysis of waste tires under different thermal process. J. Hazard. Mater. 424, 127351 (2022).
Liu, W. J., Shao, Z. G. & Xu, Y. Emission characteristics of nitrogen and sulfur containing pollutants during the pyrolysis of oily sludge with and without catalysis. J. Hazard. Mater. 401, 123820 (2021).
Bobek-Nagy, J. et al. Catalytic co-pyrolysis of packaging plastic and wood waste to achieve H2-rich syngas. Int. J. Energ. Res. 44, 10832–10845 (2020).
Winchell, L. J. et al. High-temperature technology survey and comparison among incineration, pyrolysis, and gasification systems for water resource recovery facilities. Water Environ. Res. 94, e10715 (2022).
McNamara, P. et al. Pyrolysis transports, and transforms, PFAS from biosolids to py-liquid. Environ. Sci. Water Res. Technol. 9, 386–395 (2023).
Winchell, L. J. et al. Fate of biosolids-bound PFAS through pyrolysis coupled with thermal oxidation for air emissions control. Water Environ. Res. https://doi.org/10.1002/wer.11149 (2024).
Winchell, L. J. et al. Per- and polyfluoroalkyl substances thermal destruction at water resource recovery facilities: a state of the science review. Water Environ. Res. 93, 826–843 (2021).
Sormo, E. et al. The decomposition and emission factors of a wide range of PFAS in diverse, contaminated organic waste fractions undergoing dry pyrolysis. J. Hazard. Mater. 454, 131447 (2023).
Litvanova, K., Klemetsrud, B., Xiao, F. & Kubatova, A. Investigation of real-time gaseous thermal decomposition products of representative per- and polyfluoroalkyl substances (PFAS). J. Am. Soc. Mass. Spectrom. 36, 108–118 (2025).
Stavinski, N. et al. Unraveling hidden infrared spectral signatures in PFAS thermal degradation with two-dimensional correlation spectroscopy. Environ. Sci. Technol. Lett. 12, 668–676 (2025).
Switzer, C., Pironi, P., Gerhard, J. I., Rein, G. & Torero, J. L. Self-sustaining smoldering combustion: a novel remediation process for non-aqueous-phase liquids in porous media. Environ. Sci. Technol. 43, 5871–5877 (2009).
Major, D. Demonstration of smoldering combustion treatment of PFAS-impacted investigation-derived waste. SERDP/ESTCP https://serdp-estcp.mil/projects/details/f72ddebf-217b-466f-8e68-afe857bbe983 (2019).
Chen, Y. Y., Liang, Z. R., Lin, S. R. & Huang, X. Y. Limits of sustaining a flame above smoldering woody biomass. Combust. Sci. Technol. 195, 2801–2819 (2023).
Palamba, P. & Werdhani, A. S. Experimental study of 1-D downward-opposed smoldering combustion of tropical peat and effect of excess air on the transition from smoldering to flaming. Therm. Sci. 27, 5063–5074 (2023).
Feeney, R. J., Nicotri, P. J. & Janke, D. S. Overview of thermal desorption technology. Naval Facilities Engineering Service Center https://apps.dtic.mil/sti/tr/pdf/ADA352083.pdf (1998).
Bolan, N. et al. Remediation of poly-and perfluoroalkyl substances (PFAS) contaminated soils — to mobilize or to immobilize or to degrade? J. Hazard. Mater. 401, 123892 (2021).
Sörengård, M., Lindh, A. & Ahrens, L. Thermal desorption as a high removal remediation technique for soils contaminated with per-and polyfluoroalkyl substances (PFASs). PLoS ONE 15, e0234476 (2020).
Zhang, M., Yamada, K., Bourguet, S., Guelfo, J. & Suuberg, E. M. Vapor pressure of nine perfluoroalkyl substances (PFASs) determined using the Knudsen effusion method. J. Chem. Eng. Data 65, 2332–2342 (2020).
Barranco, F. Evaluation of indirect thermal desorption coupled with thermal oxidation to treat solid PFAS-impacted investigation-derived waste. SERDP/ESTCP https://serdp-estcp.mil/projects/details/3bd7f8ca-aafc-48a6-9390-bb5d1d90ea50 (2024).
Wang, Z., Alinezhad, A., Sun, R., Xiao, F. & Pignatello, J. J. Pre- and postapplication thermal treatment strategies for sorption enhancement and reactivation of biochars for removal of per- and polyfluoroalkyl substances from water. ACS ES&T Eng. 3, 193–200 (2023).
Xu, M. G. et al. Direct measurement of fluorocarbon radicals in the thermal destruction of perfluorohexanoic acid using photoionization mass spectrometry. Sci. Adv. 11, eadt3363 (2025).
Abou-Khalil, C. et al. Enhancing the thermal mineralization of perfluorooctanesulfonate on granular activated carbon using alkali and alkaline-earth metal additives. Environ. Sci. Technol. 58, 11162–11174 (2024).
Lunde, K. E. Performance of equipment for control of fluoride emissions. Ind. Eng. Chem. 50, 293–298 (1958).
Hughey, K. D. et al. PFAS remediation: evaluating the infrared spectra of complex gaseous mixtures to determine the efficacy of thermal decomposition of PFAS. Chemosphere 362, 142631 (2024).
Carbonyl fluoride. PubChem https://pubchem.ncbi.nlm.nih.gov/compound/Carbonyl-fluoride (2023).
Altarawneh, M. A chemical kinetic model for the decomposition of perfluorinated sulfonic acids. Chemosphere 263, 128256 (2021).
Adi, M. A. & Altarawneh, M. Thermal decomposition of heptafluoropropylene-oxide-dimer acid (GenX). Chemosphere 289, 133118 (2022).
Ellis, D. A., Mabury, S. A., Martin, J. W. & Muir, D. C. G. Thermolysis of fluoropolymers as a potential source of halogenated organic acids in the environment. Nature 412, 321–324 (2001).
Sawada, H. Fluorinated peroxides. Chem. Rev. 96, 1779–1808 (1996).
Mitov, S., Panchenko, A. & Roduner, E. Comparative DFT study of non-fluorinated and perfluorinated alkyl and alkyl-peroxy radicals. Chem. Phys. Lett. 402, 485–490 (2005).
Pasteris, L., Oexler, E. V. & Staricco, E. H. Reaction of CF3 radicals with H2O and D2O. Int. J. Chem. Kinet. 15, 835–844 (1983).
Tschuikow-Roux, E. & Martell, J. E. Thermal decomposition of fluoroform in a single-pulse shock tube. I. J. Chem. Phys. 42, 2049–2056 (1965).
Baduel, C., Mueller, J. F., Rotander, A., Corfield, J. & Gomez-Ramos, M. J. Discovery of novel per- and polyfluoroalkyl substances (PFASs) at a fire fighting training ground and preliminary investigation of their fate and mobility. Chemosphere 185, 1030–1038 (2017).
Houtz, E. F., Higgins, C. P., Field, J. A. & Sedlak, D. L. Persistence of perfluoroalkyl acid precursors in AFFF-impacted groundwater and soil. Environ. Sci. Technol. 47, 8187–8195 (2013).
Nickerson, A. et al. Spatial trends of anionic, zwitterionic, and cationic PFASs at an AFFF-impacted site. Environ. Sci. Technol. 55, 313–323 (2021).
Solo-Gabriele, H. M., Jones, A. S., Lindstrom, A. B. & Lang, J. R. Waste type, incineration, and aeration are associated with per- and polyfluoroalkyl levels in landfill leachates. Waste Manag. 107, 191–200 (2020).
Munoz, G. et al. Target and nontarget screening of PFAS in biosolids, composts,and other organic waste products for land application in France. Environ. Sci. Technol. 56, 6056–6068 (2022).
Thompson, J. T. et al. Underestimation of per- and polyfluoroalkyl substances in biosolids: precursor transformation during conventional treatment. Environ. Sci. Technol. 57, 3825–3832 (2023).
Xiao, F., Hanson, R. A., Golovko, S. A., Golovko, M. Y. & Arnold, W. A. PFOA and PFOS are generated from zwitterionic and cationic precursor compounds during water disinfection with chlorine or ozone. Environ. Sci. Technol. Lett. 5, 382–388 (2018).
Liu, Z. K. et al. Accelerated degradation of perfluorosulfonates and perfluorocarboxylates by UV/sulfite plus iodide: reaction mechanisms and system efficiencies. Environ. Sci. Technol. 56, 3699–3709 (2022).
Gonda, N., Choyke, S., Schaefer, C., Higgins, C. P. & Voelker, B. Hydroxyl radical transformations of perfluoroalkyl acid (PFAA) precursors in aqueous film forming foams (AFFFs). Environ. Sci. Technol. 57, 8053–8064 (2023).
Singh, R. K. et al. Breakdown products from perfluorinated alkyl substances (PFAS) degradation in a plasma-based water treatment process. Environ. Sci. Technol. 53, 2731–2738 (2019).
Sun, R. Z., Bhat, A. P., Arnold, W. A. & Xiao, F. Investigation of transformation pathways of polyfluoroalkyl substances during chlorine disinfection. Environ. Sci. Technol. 59, 1756–1768 (2025).
Mejia-Avendano, S., Vo Duy, S., Sauve, S. & Liu, J. Generation of perfluoroalkyl acids from aerobic biotransformation of quaternary ammonium polyfluoroalkyl surfactants. Environ. Sci. Technol. 50, 9923–9932 (2016).
Jin, B., Mallula, S., Golovko, S. A., Golovko, M. Y. & Xiao, F. In vivo generation of PFOA, PFOS, and other compounds from cationic and zwitterionic per- and polyfluoroalkyl substances in a terrestrial invertebrate (Lumbricus terrestris). Environ. Sci. Technol. 54, 7378–7387 (2020).
Ruyle, B. J. et al. Nitrifying microorganisms linked to biotransformation of perfluoroalkyl sulfonamido precursors from legacy aqueous film-forming foams. Environ. Sci. Technol. 57, 5592–5602 (2023).
Cook, E. K. et al. Biological and chemical transformation of the six-carbon polyfluoroalkyl substance N-dimethyl ammonio propyl perfluorohexane sulfonamide (AmPr-FHxSA). Environ. Sci. Technol. 56, 15478–15488 (2022).
Liu, M. et al. High persistence of novel polyfluoroalkyl betaines in aerobic soils. Environ. Sci. Technol. 57, 7442–7453 (2023).
Riedel, T. P. et al. Low temperature thermal treatment of gas-phase fluorotelomer alcohols by calcium oxide. Chemosphere 272, 129859 (2021).
Wang, F., Lu, X., Li, X. Y. & Shih, K. Effectiveness and mechanisms of defluorination of perfluorinated alkyl substances by calcium compounds during waste thermal treatment. Environ. Sci. Technol. 49, 5672–5680 (2015).
Shields, E. P. & Wallace, M. A. G. Low temperature destruction of gas-phase per- and polyfluoroalkyl substances using an alumina-based catalyst. J. Air Waste Manag. Assoc. 73, 525–532 (2023).
Hao, S. et al. Hydrothermal alkaline treatment for destruction of per- and polyfluoroalkyl substances in aqueous film-forming foam. Environ. Sci. Technol. 55, 3283–3295 (2021).
Trang, B. et al. Low-temperature mineralization of perfluorocarboxylic acids. Science 377, 839–845 (2022).
Dolatabad, A. A. et al. Thermal degradation of long-chain fluorinated greenhouse gases: stability, byproducts, and remediation approaches. ACS ES&T Eng. 5, 389–401 (2024).
Weber, N. H. et al. Thermal decomposition of perfluorooctanesulfonic acid (PFOS) in the presence of water vapor. Ind. Eng. Chem. Res. 61, 15146–15155 (2022).
Rocchio, C. L., Pennell, K. D. & Goldsmith, C. F. Computational investigation of the reaction mechanism for the thermal treatment of hexafluoropropylene oxide dimer acid (GenX). J. Phys. Chem. A 129, 5343–5358 (2025).
Trimm, D. L. Catalytic combustion (review). Appl. Catal. 7, 249–282 (1983).
Lyubovsky, M. et al. Catalytic combustion over platinum group catalysts: fuel-lean versus fuel-rich operation. Catal. Today 83, 71–84 (2003).
Wang, J. L. et al. Pyrolysis of two perfluoroalkanesulfonates (PFSAs) and PFSA-laden granular activated carbon (GAC): decomposition mechanisms and the role of GAC. Environ. Sci. Technol. 58, 21850–21860 (2024).
Wang, J. L. et al. Thermal transformations of perfluorooctanoic acid (PFOA): mechanisms, volatile organofluorine emissions, and implications to thermal regeneration of granular activated carbon. J. Hazard. Mater. 479, 135737 (2024).
Gagliano, E., Sgroi, M., Falciglia, P. P., Vagliasindi, F. G. A. & Roccaro, P. Removal of poly- and perfluoroalkyl substances (PFAS) from water by adsorption: role of PFAS chain length, effect of organic matter and challenges in adsorbent regeneration. Water Res. 171, 115381 (2020).
Gagliano, E., Falciglia, P. P., Zaker, Y., Karanfil, T. & Roccaro, P. Microwave regeneration of granular activated carbon saturated with PFAS. Water Res. 198, 117121 (2021).
Li, F. et al. A concentrate-and-destroy technique for degradation of perfluorooctanoic acid in water using a new adsorptive photocatalyst. Water Res. 185, 116219 (2020).
Tian, S. T. et al. A ‘concentrate-&-destroy’ technology for enhanced removal and destruction of per- and polyfluoroalkyl substances in municipal landfill leachate. Sci. Total Environ. 791, 148124 (2021).
Zhang, Q. et al. Catalytic degradation of hexafluoropropylene oxide trimeric acid during the hydrothermal regeneration of spent activated carbon. ACS ES&T Eng. 4, 1391–1400 (2024).
Vakili, M. et al. Regeneration of exhausted adsorbents after PFAS adsorption: A critical review. J. Hazard. Mater. 471, 134429 (2024).
Ranjbar, E., Ahmadi, F. & Baghdadi, M. Regeneration of perfluorooctane sulfonic acid (PFOS) loaded granular activated carbon using organic/inorganic mixed solutions. Chem. Eng. Sci. 300, 120623 (2024).
Didenko, T. et al. Regeneration of PFAS-laden granular activated carbon by modified supercritical CO2 extraction. Chemosphere 370, 143986 (2025).
Sun, R. Z., Sasi, P. C., Alinezhad, A. & Xiao, F. Sorptive removal of per- and polyfluoroalkyl substances (PFAS) in organic-free water, surface water, and landfill leachate and thermal reactivation of spent sorbents. J. Hazard. Mater. Adv. 10, 100311 (2023).
Wen, J. Y., Neha, S., Biller, P., Kristensen, K. & Vergeynst, L. Detection of volatile hydroperfluoroalkanes during hydrothermal liquefaction of perfluoroalkyl carboxylic acids at circumneutral pH. J. Hazard. Mater. 476, 134955 (2024).
Smith, A. H. Infant exposure assessment for breast milk dioxins and furans derived from waste incineration emissions. Risk Anal. 7, 347–353 (1987).
Mininni, G., Sbrilli, A., Guerriero, E. & Rotatori, M. Dioxins and furans formation in pilot incineration tests of sewage sludge spiked with organic chlorine. Chemosphere 54, 1337–1350 (2004).
Cunliffe, A. M. & Williams, P. T. De-novo formation of dioxins and furans and the memory effect in waste incineration flue gases. Waste Manag. 29, 739–748 (2009).
Ajay, S. V. & Prathish, K. P. Dioxins emissions from bio-medical waste incineration: a systematic review on emission factors, inventories, trends and health risk studies. J. Hazard. Mater. 465, 133384 (2024).
PFAS Thermal Treatment Database. United States Environmental Protection Agency https://www.epa.gov/chemical-research/pfas-thermal-treatment-database-pfastt (2024).
Acknowledgements
This work was supported by the University of Missouri’s MizzouForward programme, the US Department of Defense Strategic Environmental Research and Development Program (ER21-1019, ER21-1234, ER22-4014 and ER24-4073), the Environmental Research and Education Foundation, and the US National Science Foundation (CBET 2320966).
Author information
Authors and Affiliations
Contributions
A.A.D. contributed to the figure and manuscript preparation. F.X. contributed to the overall conception and design of the Review, figure development and manuscript writing. All authors contributed to reviewing the literature, manuscript preparation, providing critical feedback and revising the manuscript.
Corresponding author
Ethics declarations
Competing interests
L.W. is an employee of Brown and Caldwell, an environmental engineering and consulting firm specializing in water and wastewater treatment and environmental remediation; Brown and Caldwell is not an equipment vendor. M.A. is an employee of AECOM Technical Services, which is not an equipment vendor. K.P. reports a relationship with Pennell Environmental LLC for consulting and expert advice, which is not an equipment vendor. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Clean Technology thanks Takashi Fujimori; Hans-Joachim Gehrmann, who co-reviewed with Vanessa Nuredin and Anna Holfelder; and Igor Novosselov, who co-reviewed with Almond Lau, for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Arhami Dolatabad, A., Blotevogel, J., Ateia, M. et al. PFAS thermal treatment approaches and enhancement. Nat. Rev. Clean Technol. 2, 38–53 (2026). https://doi.org/10.1038/s44359-025-00122-5
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s44359-025-00122-5