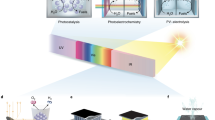
Abstract
Solar fuel technologies use sunlight to synthesize value-added molecules that can be used as renewable fuels or chemical feedstock. They share the common principle of converting and storing solar and other renewable energy in chemical bonds, but differ in the harvesting mechanisms, catalytic processes and systems used. These distinctions lead to different advantages, challenges, maturity and deployability prospects. In this Perspective, we provide a cross-disciplinary view of five major solar fuel platforms — photocatalysis, photovoltaic-driven electrolysis (PV + EC), photoelectrochemical, photothermal and plasmonic catalysis — to identify transferable insights and design principles. The wide span of solar-to-hydrogen efficiency (typically 0.1–15%) and levelized cost of hydrogen (US$2–30 kg−1 H2) depending on the system reflect both mechanistic limitations and system-level constraints that keep performance below theoretical limits. Common bottlenecks emerge, including spectral mismatch, charge-management and heat-management losses, stability in harsh operating environments and dependence on critical materials. At the same time, shared design principles — such as defect and facet engineering, multi-absorber architectures, plasmonic and photothermal enhancement, interface stabilization and catalyst–reactor co-design — offer transferable strategies capable of improving performance across platforms. Together, these insights provide a transversal unifying vision on how advances in one solar fuel technology can accelerate progress in others and inform pathways towards scalable, efficient and economically viable solar fuel production.
Key points
-
Solar fuel platforms operate under distinct principles — photonic, electrochemical, thermal or hybrid — but face convergent challenges in efficiency, stability and reactor design, making comparative evaluation both feasible and necessary.
-
Levelized cost of hydrogen estimates range widely, from <$5 kg−1 H2 for optimized photovoltaic-driven electrolysis (PV + EC) to >$20 kg−1 H2 for early-stage plasmonic systems. Efficiency, capital expenditure (CAPEX) and operating expenditure (OPEX) balance and material costs are primary cost drivers.
-
Solar-to-hydrogen efficiencies span from ~0.1% to 30%; however, overall viability is more tightly linked to durability, system simplicity and integration potential than to efficiency alone.
-
Cross-platform developments can accelerate innovation. Photoelectrochemical (PEC) systems benefit from PV-derived absorbers and scalable encapsulation methods; PV + EC and photocatalytic systems can adopt the spatial catalyst separation of PEC to enhance selectivity and modularity; and advances in thermal integration, local heating and spectrum shaping in plasmonic and photothermal systems could benefit low-temperature PEC and photocatalytic configurations.
-
Scalable progress relies on platform-specific strengths with photocatalysis and PEC emphasizing material simplicity and integration; PV + EC leveraging commercial maturity; and plasmonic and photothermal systems offering spectral and thermal control.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Perez, M. & Perez, R. Update 2022 — a fundamental look at supply side energy reserves for the planet. Sol. Energy Adv. 2, 100014 (2022).
Lewis, N. S. Research opportunities to advance solar energy utilization. Science 351, 356 (2016).
Wang, Q., Pornrungroj, C., Linley, S. & Reisner, E. Strategies to improve light utilization in solar fuel synthesis. Nat. Energy 7, 13–24 (2022).
Lv, J. et al. Solar utilization beyond photosynthesis. Nat. Rev. Chem. 7, 91–105 (2023).
National Renewable Energy Laboratory. Life Cycle Green House Emissions From Photovoltaics (NREL, 2012).
Frischknecht, R. Environmental Life Cycle Assessment of Electricity from PV Systems — 2021 data update (IEA, 2022).
Segev, G. et al. The 2022 solar fuels roadmap. J. Phys. D Appl. Phys. 55, 323003 (2022).
Kim, J. H., Hansora, D., Sharma, P., Jang, J. W. & Lee, J. S. Toward practical solar hydrogen production — an artificial photosynthetic leaf-to-farm challenge. Chem. Soc. Rev. 48, 1908–1971 (2019).
Song, H., Luo, S., Huang, H., Deng, B. & Ye, J. Solar-driven hydrogen production: recent advances, challenges, and future perspectives. ACS Energy Lett. 7, 1043–1065 (2022).
Comer, B. M. et al. Prospects and challenges for solar fertilizers. Joule 3, 1578–1605 (2019).
Ardo, S. et al. Pathways to electrochemical solar-hydrogen technologies. Energy Environ. Sci. 11, 2768–2783 (2018).
Götz, M. et al. Renewable power-to-gas: a technological and economic review. Renew. Energy 85, 1371–1390 (2016).
Jia, J. et al. Solar water splitting by photovoltaic-electrolysis with a solar-to-hydrogen efficiency over 30%. Nat. Commun. 7, 13237 (2016).
Schäppi, R. et al. Drop-in fuels from sunlight and air. Nature 601, 63–68 (2022).
Yaghoubi, S. et al. Photocatalysts for solar energy conversion: recent advances and environmental applications. Renew. Sustain. Energy Rev. 200, 114538 (2024).
Luo, S., Ren, X., Lin, H., Song, H. & Ye, J. Plasmonic photothermal catalysis for solar-to-fuel conversion: current status and prospects. Chem. Sci. 12, 5701–5719 (2021).
Wienhold, M., Molloy, J. J. & Gilmour, R. Advances in the E → Z isomerization of alkenes using small molecule photocatalysts. Chem. Rev. 122, 2650–2694 (2022).
Chen, R., Ni, C., Zhu, J., Fan, F. & Li, C. Surface photovoltage microscopy for mapping charge separation on photocatalyst particles. Nat. Protoc. 19, 2250–2282 (2024).
Yuan, C. et al. Light-induced CoOX surface reconstruction in hollow heterostructure for durable photocatalytic seawater splitting. Nat. Commun. 16, 6607 (2025).
Manning, C. G. Technology readiness levels. NASA https://www.nasa.gov/directorates/somd/space-communications-navigation-program/technology-readiness-levels (2023).
Naldoni, A., Shalaev, V. M. & Brongersma, M. L. Applying plasmonics to a sustainable future. Science 356, 908–909 (2017).
Takata, T. et al. Photocatalytic water splitting with a quantum efficiency of almost unity. Nature 581, 411–414 (2020).
Nishiyama, H. et al. Photocatalytic solar hydrogen production from water on a 100-m2 scale. Nature 598, 304–307 (2021).
Zoller, S. et al. A solar tower fuel plant for the thermochemical production of kerosene from H2O and CO2. Joule 6, 1606–1616 (2022).
Cheng, W. H. et al. Monolithic photoelectrochemical device for direct water splitting with 19% efficiency. ACS Energy Lett. 3, 1795–1800 (2018).
Holmes-Gentle, I., Tembhurne, S., Suter, C. & Haussener, S. Kilowatt-scale solar hydrogen production system using a concentrated integrated photoelectrochemical device. Nat. Energy 8, 586–596 (2023).
Nielander, A. C., Shaner, M. R., Papadantonakis, K. M., Francis, S. A. & Lewis, N. S. A taxonomy for solar fuels generators. Energy Environ. Sci. 8, 16–25 (2015).
Zhao, D. et al. Boron-doped nitrogen-deficient carbon nitride-based Z-scheme heterostructures for photocatalytic overall water splitting. Nat. Energy 6, 388–397 (2021).
Li, A. et al. Three-phase photocatalysis for the enhanced selectivity and activity of CO2 reduction on a hydrophobic surface. Angew. Chem. Int. Ed. 58, 14549–14555 (2019).
Rahimi, F. A., Dey, S., Verma, P. & Maji, T. K. Photocatalytic CO2 reduction based on a Re(I)-integrated conjugated microporous polymer: role of a sacrificial electron donor in product selectivity and efficiency. ACS Catal. 13, 5969–5978 (2023).
Cheng, N. et al. Au-nanoparticle-loaded graphitic carbon nitride nanosheets: green photocatalytic synthesis and application toward the degradation of organic pollutants. ACS Appl. Mater. Interfaces 5, 6815–6819 (2013).
Zhou, X. et al. Photocatalytic dehydrogenative C–C coupling of acetonitrile to succinonitrile. Nat. Commun. 13, 4379 (2022).
Kang, L. et al. Light-driven propane dehydrogenation by a single-atom catalyst under near-ambient conditions. Nat. Chem. 17, 890–896 (2025).
Sun, R. et al. Regulation of Pd single-atom coordination for enhanced photocatalytic oxidation of toluene to benzaldehyde. Nat. Synth. 4, 965–975 (2025).
Gisbertz, S., Reischauer, S. & Pieber, B. Overcoming limitations in dual photoredox/nickel-catalysed C–N cross-couplings due to catalyst deactivation. Nat. Catal. 3, 611–620 (2020).
Li, J., Chang, J., Li, M. & Han, Q. Visible-light-driven C−N bond formation by a hexanickel cluster substituted polyoxometalate-based photocatalyst. Inorg. Chem. 60, 10022–10029 (2021).
Kong, D. et al. Recent advances in visible light-driven water oxidation and reduction in suspension systems. Mater. Today 21, 897–924 (2018).
Wang, W. N. et al. Size and structure matter: enhanced CO2 photoreduction efficiency by size-resolved ultrafine Pt nanoparticles on TiO2 single crystals. J. Am. Chem. Soc. 134, 11276–11281 (2012).
Ola, O. & Mercedes Maroto-Valer, M. Role of catalyst carriers in CO2 photoreduction over nanocrystalline nickel loaded TiO2-based photocatalysts. J. Catal. 309, 300–308 (2014).
Tang, L., Wang, Z. L., Wan, H. L., He, Y. H. & Guan, Z. Visible-light-induced Beckmann rearrangement by organic photoredox catalysis. Org. Lett. 22, 6182–6186 (2020).
Schneider, V., Polonskyi, O., Strunskus, T., Elbahri, M. & Faupel, F. Light-induced conductance switching in photomechanically active carbon nanotube-polymer composites. Sci. Rep. 7, 2–10 (2017).
Huang, X. et al. Direct visible-light-excited asymmetric Lewis acid catalysis of intermolecular [2+2] photocycloadditions. J. Am. Chem. Soc. 139, 9120–9123 (2017).
Kübler, J. A., Pfund, B. & Wenger, O. S. Zinc(II) complexes with triplet charge-transfer excited states enabling energy-transfer catalysis, photoinduced electron transfer, and upconversion. JACS Au 2, 2367–2380 (2022).
Dai, L. et al. Diastereo- and atroposelective synthesis of N-arylpyrroles enabled by light-induced phosphoric acid catalysis. Nat. Commun. 14, 4813 (2023).
Liu, G., Wang, L., Yang, H. G., Cheng, H. M. & Lu, G. Q. Titania-based photocatalysts — crystal growth, doping and heterostructuring. J. Mater. Chem. 20, 831–843 (2010).
Kudo, A. Z-scheme photocatalyst systems for water splitting under visible light irradiation. MRS Bull. 36, 32–38 (2011).
Yong, X. & Schoonen, M. A. A. The absolute energy positions of conduction and valence bands of selected semiconducting minerals. Am. Mineral. 85, 543–556 (2000).
Chen, S. et al. Semiconductor-based photocatalysts for photocatalytic and photoelectrochemical water splitting: will we stop with photocorrosion? J. Mater. Chem. A 8, 2286–2322 (2020).
Chen, Y. et al. Facet-engineered TiO2 drives photocatalytic activity and stability of supported noble metal clusters during H2 evolution. Nat. Commun. 14, 6165 (2023).
Wang, Q. et al. Scalable water splitting on particulate photocatalyst sheets with a solar-to-hydrogen energy conversion efficiency exceeding 1%. Nat. Mater. 15, 611–615 (2016).
Lyu, H. et al. An Al-doped SrTiO3 photocatalyst maintaining sunlight-driven overall water splitting activity for over 1000 h of constant illumination. Chem. Sci. 10, 3196–3201 (2019).
Lin, L. et al. Efficient and stable visible-light-driven Z-scheme overall water splitting using an oxysulfide H2 evolution photocatalyst. Nat. Commun. 15, 397 (2024).
Brongersma, M. L., Halas, N. J. & Nordlander, P. Plasmon-induced hot carrier science and technology. Nat. Nanotechnol. 10, 25–34 (2015).
Aslam, U., Rao, V. G., Chavez, S. & Linic, S. Catalytic conversion of solar to chemical energy on plasmonic metal nanostructures. Nat. Catal. 1, 656–665 (2018).
Yuan, L. et al. Plasmonic photocatalysis with chemically and spatially specific antenna-dual reactor complexes. ACS Nano 16, 17365–17375 (2022).
Robatjazi, H. et al. Plasmon-induced selective carbon dioxide conversion on earth-abundant aluminum-cuprous oxide antenna-reactor nanoparticles. Nat. Commun. 8, 27 (2017).
Guo, Y., Xu, Z., Curto, A. G., Zeng, Y. J. & Van Thourhout, D. Plasmonic semiconductors: materials, tunability and applications. Prog. Mater. Sci. 138, 101158 (2023).
Singh, S. et al. Surface plasmon-enhanced photo-driven CO2 hydrogenation by hydroxy-terminated nickel nitride nanosheets. Nat. Commun. 14, 2551 (2023).
Aslam, U., Chavez, S. & Linic, S. Controlling energy flow in multimetallic nanostructures for plasmonic catalysis. Nat. Nanotechnol. 12, 1000–1005 (2017).
Kim, M., Lin, M., Son, J., Xu, H. & Nam, J. M. Hot-electron-mediated photochemical reactions: principles, recent advances, and challenges. Adv. Opt. Mater. 5, 1–21 (2017).
Zhuo, X., Vila-Liarte, D., Wang, S., Jimenez de Aberasturi, D. & Liz-Marzán, L. M. Coated chiral plasmonic nanorods with enhanced structural stability. Chem. Mater. 35, 5689–5698 (2023).
Coviello, V., Forrer, D. & Amendola, V. Recent developments in plasmonic alloy nanoparticles: synthesis, modelling, properties and applications. ChemPhysChem 23, e202200136 (2022).
Krekeler, T. et al. Unprecedented thermal stability of plasmonic titanium nitride films up to 1400 °C. Adv. Opt. Mater. 9, 2100323 (2021).
Clavero, C. Plasmon-induced hot-electron generation at nanoparticle/metal-oxide interfaces for photovoltaic and photocatalytic devices. Nat. Photon. 8, 95–103 (2014).
Sharma, G. et al. Pt-doped Ru nanoparticles loaded on ‘black gold’ plasmonic nanoreactors as air stable reduction catalysts. Nat. Commun. 15, 713 (2024).
Cao, M., Shao, S., Ji, W. & Fan, X. Enhanced plasmonic photocatalytic performance of C doped TiN nanocrystals through ultrathin carbon layers. J. Environ. Manage. 345, 118826 (2023).
da Silva, A. G. M., Rodrigues, T. S., Wang, J. & Camargo, P. H. C. Plasmonic catalysis with designer nanoparticles. Chem. Commun. 58, 2055–2074 (2022).
Wang, D. et al. Spatial and temporal nanoscale plasmonic heating quantified by thermoreflectance. Nano Lett. 19, 3796–3803 (2019).
Setoura, K., Tamura, M., Oshikiri, T. & Iida, T. Switching nanoscale temperature fields with high-order plasmonic modes in transition metal nanorods. RSC Adv. 13, 34489–34496 (2023).
Chen, C., Kuang, Y. & Hu, L. Challenges and opportunities for solar evaporation. Joule 3, 683–718 (2019).
Wells, M. P. et al. Temperature stability of thin film refractory plasmonic materials. Opt. Express 26, 15726 (2018).
Shi, L. et al. SiC-C composite as a highly stable and easily regenerable photothermal material for practical water evaporation. ACS Sustain. Chem. Eng. 6, 8192–8200 (2018).
Cui, X. et al. Photothermal nanomaterials: a powerful light-to-heat converter. Chem. Rev. 123, 6891–6952 (2023).
Xu, C. et al. Fibrous aerogels for solar vapor generation. Front. Chem. 10, 1–19 (2022).
Farid, M. U., Kharraz, J. A., Wang, P. & An, A. K. High-efficiency solar-driven water desalination using a thermally isolated plasmonic membrane. J. Clean. Prod. 271, 122684 (2020).
Das, S. et al. Core–shell structured catalysts for thermocatalytic, photocatalytic, and electrocatalytic conversion of CO2. Chem. Soc. Rev. 49, 2937–3004 (2020).
Xiong, P. et al. Synthesis of core@shell catalysts guided by Tammann temperature. Nat. Commun. 15, 420 (2024).
Song, C., Wang, Z., Yin, Z., Xiao, D. & Ma, D. Principles and applications of photothermal catalysis. Chem. Catal. 2, 52–83 (2022).
Zi, Y., Hu, Y., Pu, J., Wang, M. & Huang, W. Recent progress in interface engineering of nanostructures for photoelectrochemical energy harvesting applications. Small 19, 2208274 (2023).
Vilanova, A., Dias, P., Lopes, T. & Mendes, A. The route for commercial photoelectrochemical water splitting: a review of large-area devices and key upscaling challenges. Chem. Soc. Rev. 53, 2388–2434 (2024).
Taseska, T. et al. Analysis of the scale of global human needs and opportunities for sustainable catalytic technologies. Top. Catal. 66, 338–374 (2023).
Jiang, C., Moniz, S. J. A., Wang, A., Zhang, T. & Tang, J. Photoelectrochemical devices for solar water splitting-materials and challenges. Chem. Soc. Rev. 46, 4645–4660 (2017).
Lim, H. et al. High performance III–V photoelectrodes for solar water splitting via synergistically tailored structure and stoichiometry. Nat. Commun. 10, 3388 (2019).
Chem, J. M. Plasmon-dominated photoelectrodes for solar water splitting. J. Mater. Chem. A 5, 4233–4253 (2017).
Qian, K. et al. Surface plasmon-driven water reduction: gold nanoparticle size matters. J. Am. Chem. Soc. 136, 9842–9845 (2014).
Ghobadi, T. G. U., Ghobadi, A., Ozbay, E. & Karadas, F. Strategies for plasmonic hot-electron-driven photoelectrochemical water splitting. ChemPhotoChem 2, 161–182 (2018).
Peerakiatkhajohn, P. et al. A hybrid photoelectrode with plasmonic Au@TiO2 nanoparticles for enhanced photoelectrochemical water splitting. J. Mater. Chem. A 3, 20127–20133 (2015).
Ueno, K., Mor, Y. & Osh, T. Plasmon-enhanced water splitting utilizing the heterojunction synergistic effect between SrTiO3 and rutile-TiO2. Chem. Lett. 44, 618–620 (2015).
Sivula, K. & van de Krol, R. Semiconducting materials for photoelectrochemical energy conversion. Nat. Rev. Mater. 1, 15010 (2016).
Tang, S., Qiu, W., Xiao, S., Tong, Y. & Yang, S. Harnessing hierarchical architectures to trap light for efficient photoelectrochemical cells. Energy Environ. Sci. 13, 660–684 (2020).
Shockley, W. & Queisser, H. J. Detailed balance limit of efficiency of p–n junction solar cells. J. Appl. Phys. 32, 510–519 (1961).
National Renewable Energy Laboratory. Best research-cell efficiency chart. NREL https://www.nrel.gov/pv/cell-efficiency (2025).
Pham, D. P., Lee, S. & Yi, J. Photovoltaic partner selection for high-efficiency photovoltaic-electrolytic water splitting systems: brief review and perspective. Silicon 14, 753–760 (2022).
Wang, T., Cao, X. & Jiao, L. PEM water electrolysis for hydrogen production: fundamentals, advances, and prospects. Carb. Neutrality 1, 21 (2022).
Rabinowitz, J. A. & Kanan, M. W. The future of low-temperature carbon dioxide electrolysis depends on solving one basic problem. Nat. Commun. 11, 5231 (2020).
Leonzio, G., Hankin, A. & Shah, N. CO2 electrochemical reduction: a state-of-the-art review with economic and environmental analyses. Chem. Eng. Res. Des. 208, 934–955 (2024).
Carmo, M. & Fritz, D. L. A comprehensive review on PEM water electrolysis. Int. J. Hydrogen Energy 38, 4901–4934 (2013).
Cheng, W. H. et al. CO2 reduction to CO with 19% efficiency in a solar-driven gas diffusion electrode flow cell under outdoor solar illumination. ACS Energy Lett. 5, 470–476 (2020).
Gao, J. et al. Solar reduction of carbon dioxide on copper-tin electrocatalysts with energy conversion efficiency near 20%. Nat. Commun. 13, 5898 (2022).
Ma, G. et al. Mn-doped NiCoP nanopin arrays as high-performance bifunctional electrocatalysts for sustainable hydrogen production via overall water splitting. Nano Energy 115, 108679 (2023).
Xu, H. et al. Highly selective electrocatalytic CO2 reduction to ethanol by metallic clusters dynamically formed from atomically dispersed copper. Nat. Energy 5, 623–632 (2020).
Fang, Y. et al. Graphdiyne interface engineering: highly active and selective ammonia synthesis. Angew. Chem. Int. Ed. 59, 13021–13027 (2020).
Vequizo, J. J. M. et al. Trapping-induced enhancement of photocatalytic activity on brookite TiO2 powders: comparison with anatase and rutile TiO2 powders. ACS Catal. 7, 2644–2651 (2017).
Lian, Z. et al. Photogenerated hole traps in metal-organic-framework photocatalysts for visible-light-driven hydrogen evolution. Commun. Chem. 5, 93 (2022).
Zhang, L. et al. Visible-light-driven non-oxidative dehydrogenation of alkanes at ambient conditions. Nat. Energy 7, 1042–1051 (2022).
Wang, J. et al. Regulating the metallic Cu–Ga bond by S vacancy for improved photocatalytic CO2 reduction to C2H4. Adv. Funct. Mater. 33, 2213901 (2023).
Zhang, Y. et al. H2O2 generation from O2 and H2O on a near-infrared absorbing porphyrin supramolecular photocatalyst. Nat. Energy 8, 361–371 (2023).
Teitsworth, T. S. et al. Water splitting with silicon p–i–n superlattices suspended in solution. Nature 614, 270–274 (2023).
Li, Z. et al. Engineered disorder in CO2 photocatalysis. Nat. Commun. 13, 7205 (2022).
Xu, R. et al. Tandem photocatalysis of CO2 to C2H4 via a synergistic rhenium-(I) bipyridine/copper-porphyrinic triazine framework. J. Am. Chem. Soc. 145, 8261–8270 (2023).
Liu, J. et al. Metal-free efficient photocatalyst for stable visible water splitting via a two-electron pathway. Science 347, 970–974 (2015).
Zhu, Y., Xue, Y., Wang, Z. & Chao, D. Robust noble metal-free photoconversion of CO2 to CO with a molecule/MOF hybrid catalyst for straightforward two-chamber organic carbonylation. ACS Catal. 15, 3546–3557 (2025).
Xia, Y. S. et al. Tandem utilization of CO2 photoreduction products for the carbonylation of aryl iodides. Nat. Commun. 13, 2964 (2022).
Zhang, Y. et al. Internal quantum efficiency higher than 100% achieved by combining doping and quantum effects for photocatalytic overall water splitting. Nat. Energy 8, 504–514 (2023).
Fan, Y. et al. Highly-efficient overall water splitting in 2D Janus group-III chalcogenide multilayers: the roles of intrinsic electric filed and vacancy defects. Sci. Bull. 65, 27–34 (2020).
Fu, C. F. et al. Intrinsic electric fields in two-dimensional materials boost the solar-to-hydrogen efficiency for photocatalytic water splitting. Nano Lett. 18, 6312–6317 (2018).
Xia, C. et al. Universality of electronic characteristics and photocatalyst applications in the two-dimensional Janus transition metal dichalcogenides. Phys. Rev. B 98, 1–8 (2018).
Tu, J., Wu, W., Lei, X. & Li, P. The SWSe-BP vdW heterostructure as a promising photocatalyst for water splitting with power conversion efficiency of 19.4%. ACS Omega 7, 37061–37069 (2022).
Zhou, L. et al. Light-driven methane dry reforming with single atomic site antenna-reactor plasmonic photocatalysts. Nat. Energy 5, 61–70 (2020).
Kang, Y. et al. Effect of crystal facets in plasmonic catalysis. Nat. Commun. 15, 3923 (2024).
Dhiman, M. et al. Plasmonic colloidosomes of black gold for solar energy harvesting and hotspots directed catalysis for CO2 to fuel conversion. Chem. Sci. 10, 6594–6603 (2019).
Verma, R. et al. Nickel-laden dendritic plasmonic colloidosomes of black gold: forced plasmon mediated photocatalytic CO2 hydrogenation. ACS Nano 17, 4526–4538 (2023).
Zhou, L. et al. Quantifying hot carrier and thermal contributions in plasmonic photocatalysis. Science 364, 69–72 (2019).
Sivan, Y., Baraban, J., Un, I. W. & Dubi, Y. Comment on ‘Quantifying hot carrier and thermal contributions in plasmonic photocatalysis’. Science 364, 1–3 (2019).
Zhan, C. et al. Disentangling charge carrier from photothermal effects in plasmonic metal nanostructures. Nat. Commun. 10, 2671 (2019).
Dubi, Y. & Sivan, Y. ‘Hot’ electrons in metallic nanostructures — non-thermal carriers or heating? Light Sci. Appl. 8, 89 (2019).
Herran, M. et al. Plasmonic bimetallic two-dimensional supercrystals for H2 generation. Nat. Catal. 6, 1205–1214 (2023).
Liu, S. et al. Quantifying the distinct role of plasmon enhancement mechanisms in prototypical antenna-reactor photocatalysts. Nat. Commun. 16, 2245 (2025).
Verma, R., Sharma, G. & Polshettiwar, V. The paradox of thermal vs. non-thermal effects in plasmonic photocatalysis. Nat. Commun. 15, 7974 (2024).
Cai, M. et al. Greenhouse-inspired supra-photothermal CO2 catalysis. Nat. Energy 6, 807–814 (2021).
Chen, G. et al. Alumina-supported CoFe alloy catalysts derived from layered-double-hydroxide nanosheets for efficient photothermal CO2 hydrogenation to hydrocarbons. Adv. Mater. 30, 1–8 (2018).
Xu, Y. F. et al. High-performance light-driven heterogeneous CO2 catalysis with near-unity selectivity on metal phosphides. Nat. Commun. 11, 5149 (2020).
Rossell, M. D. et al. Direct evidence of surface reduction in monoclinic BiVO4. Chem. Mater. 27, 3593–3600 (2015).
Yu, G., Gao, J., Hummelen, J. C., Wudi, F. & Heeger, A. J. Polymer photovoltaic cells: enhanced efficiencies via a network of internal donor–acceptor heterojunctions. Science 270, 1789–1791 (1995).
Walter, M. G. et al. Solar water splitting cells. Chem. Rev. 110, 6446–6473 (2010).
Cho, H. H. et al. A semiconducting polymer bulk heterojunction photoanode for solar water oxidation. Nat. Catal. 4, 431–438 (2021).
Daboczi, M. et al. Enhanced solar water oxidation and unassisted water splitting using graphite-protected bulk heterojunction organic photoactive layers. Nat. Energy 10, 581–591 (2025).
Zhang, B. et al. Homogeneously dispersed multimetal oxygen-evolving catalysts. Science 352, 333–337 (2016).
Rossi, K. & Buonsanti, R. Shaping copper nanocatalysts to steer selectivity in the electrochemical CO2 reduction reaction. Acc. Chem. Res. 55, 629–637 (2022).
Nitopi, S. et al. Progress and perspectives of electrochemical CO2 reduction on copper in aqueous electrolyte. Chem. Rev. 119, 7610–7672 (2019).
Wilsey, M. K., Cox, C. P., Forsythe, R. C., Mccarney, L. R. & Müller, A. M. Selective CO2 reduction towards a single upgraded product: a minireview on multi-elemental copper-free electrocatalysts. Catal. Sci. Technol. 11, 416–424 (2021).
Hammer, B. & Nørskov, J. K. Theoretical surface science and catalysis — calculations and concepts. Adv. Catal. 45, 71–129 (2000).
Nørskov, J. K. et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 108, 17886–17892 (2004).
Chen, H. et al. Promotion of electrochemical CO2 reduction to ethylene on phosphorus-doped copper nanocrystals with stable Cuδ+ sites. Appl. Surf. Sci. 544, 148965 (2021).
Wu, H. et al. Selective and energy-efficient electrosynthesis of ethylene from CO2 by tuning the valence of Cu catalysts through aryl diazonium functionalization. Nat. Energy 9, 422–433 (2024).
Jeon, H. S. et al. Operando insight into the correlation between the structure and composition of CuZn nanoparticles and their selectivity for the electrochemical CO2 reduction. J. Am. Chem. Soc. 141, 19879–19887 (2019).
Lei, Q. et al. Structural evolution and strain generation of derived-Cu catalysts during CO2 electroreduction. Nat. Commun. 13, 4857 (2022).
Okatenko, V. et al. Alloying as a strategy to boost the stability of copper nanocatalysts during the electrochemical CO2 reduction reaction. J. Am. Chem. Soc. 145, 5370–5383 (2023).
Golovanova, V. et al. Effects of solar irradiation on thermally driven CO2 methanation using Ni/CeO2-based catalyst. Appl. Catal. B Environ. 291, 120038 (2021).
Tan, T. H. et al. Unlocking the potential of the formate pathway in the photo-assisted Sabatier reaction. Nat. Catal. 3, 1034–1043 (2020).
Shoji, S. et al. Photocatalytic uphill conversion of natural gas beyond the limitation of thermal reaction systems. Nat. Catal. 3, 148–153 (2020).
Hu, C. et al. Near-infrared-featured broadband CO2 reduction with water to hydrocarbons by surface plasmon. Nat. Commun. 14, 221 (2023).
Zhang, X. et al. Product selectivity in plasmonic photocatalysis for carbon dioxide hydrogenation. Nat. Commun. 8, 14542 (2017).
Lou, M. et al. Direct H2S decomposition by plasmonic photocatalysis: efficient remediation plus sustainable hydrogen production. ACS Energy Lett. 7, 3666–3674 (2022).
Qi, J. et al. Dynamic control of elementary step energetics via pulsed illumination enhances photocatalysis on metal nanoparticles. ACS Energy Lett. 5, 3518–3525 (2020).
Boerigter, C., Campana, R., Morabito, M. & Linic, S. Evidence and implications of direct charge excitation as the dominant mechanism in plasmon-mediated photocatalysis. Nat. Commun. 7, 10545 (2016).
Yu, S., Wilson, A. J., Heo, J. & Jain, P. K. Plasmonic control of multi-electron transfer and C–C coupling in visible-light-driven CO2 reduction on Au nanoparticles. Nano Lett. 18, 2189–2194 (2018).
Zhang, W., Wang, L., Wang, K., Khan, M. U. & Wang, M. Integration of photothermal effect and heat insulation to efficiently reduce reaction temperature of CO2 hydrogenation. Small 13, 1–5 (2017).
Baldi, A. & Askes, S. H. C. Pulsed photothermal heterogeneous catalysis. ACS Catal. 13, 3419–3432 (2023).
Wang, X. et al. Pivotal role of reversible NiO6 geometric conversion in oxygen evolution. Nature 611, 702–708 (2022).
Xiang, F. et al. Light-induced quantum reconfiguration of oxyhydroxides for photoanodes with 4.24% efficiency and stability beyond 250 hours. Adv. Mater. 36, 2405478 (2024).
Wang, J. et al. Light-induced dynamic activation of copper/silicon interface for highly selective carbon dioxide reduction. Angew. Chem. Int. Ed. 63, e202403333 (2024).
Bergmann, A. & Roldan Cuenya, B. Operando insights into nanoparticle transformations during catalysis. ACS Catal. 9, 10020–10043 (2019).
Möller, T. et al. Electrocatalytic CO2 reduction on CuOx nanocubes: tracking the evolution of chemical state, geometric structure, and catalytic selectivity using operando spectroscopy. Angew. Chem. Int. Ed. 59, 17974–17983 (2020).
Wang, W., Duan, J., Liu, Y. & Zhai, T. Structural reconstruction of catalysts in electroreduction reaction: identifying, understanding, and manipulating. Adv. Mater. 34, 2110699 (2022).
Mefford, J. T. et al. Correlative operando microscopy of oxygen evolution electrocatalysts. Nature 593, 67–73 (2021).
Zhan, C. et al. Revealing the CO coverage-driven C–C coupling mechanism for electrochemical CO2 reduction on Cu2O nanocubes via operando Raman spectroscopy. ACS Catal. 11, 7694–7701 (2021).
McCrum, I. T. & Koper, M. T. M. The role of adsorbed hydroxide in hydrogen evolution reaction kinetics on modified platinum. Nat. Energy 5, 891–899 (2020).
Li, K. et al. Enhancement of lithium-mediated ammonia synthesis by addition of oxygen. Science 374, 1593–1597 (2021).
Li, F. et al. Molecular tuning of CO2-to-ethylene conversion. Nature 577, 509–513 (2020).
Nam, D. H. et al. Molecular enhancement of heterogeneous CO2 reduction. Nat. Mater. 19, 266–276 (2020).
Liang, Y. et al. Enhancement of electrocatalytic oxygen evolution by chiral molecular functionalization of hybrid 2D electrodes. Nat. Commun. 13, 3356 (2022).
Kaminsky, C. J., Weng, S., Wright, J. & Surendranath, Y. Adsorbed cobalt porphyrins act like metal surfaces in electrocatalysis. Nat. Catal. 5, 430–442 (2022).
Zhou, P. et al. Solar-to-hydrogen efficiency of more than 9% in photocatalytic water splitting. Nature 613, 66–70 (2023).
Zhou, H. et al. Increasing the efficiency of photocatalytic reactions via surface microenvironment engineering. J. Am. Chem. Soc. 142, 2738–2743 (2020).
Li, Y. et al. Electrolyte-assisted polarization leading to enhanced charge separation and solar-to-hydrogen conversion efficiency of seawater splitting. Nat. Catal. 7, 77–88 (2024).
Yu, S. & Jain, P. K. Plasmonic photosynthesis of C1–C3 hydrocarbons from carbon dioxide assisted by an ionic liquid. Nat. Commun. 10, 2022 (2019).
Corson, E. R. et al. In situ ATR-SEIRAS of carbon dioxide reduction at a plasmonic silver cathode. J. Am. Chem. Soc. 142, 11750–11762 (2020).
Li, X., Zhang, X., Everitt, H. O. & Liu, J. Light-induced thermal gradients in ruthenium catalysts significantly enhance ammonia production. Nano Lett. 19, 1706–1711 (2019).
Guo, S., Li, X., Li, J. & Wei, B. Boosting photocatalytic hydrogen production from water by photothermally induced biphase systems. Nat. Commun. 12, 1343 (2021).
Andrei, V. et al. Long-term solar water and CO2 splitting with photoelectrochemical BiOI–BiVO4 tandems. Nat. Mater. 21, 864–868 (2022).
Ros, C. et al. Turning earth abundant kesterite-based solar cells into efficient protected water-splitting photocathodes. ACS Appl. Mater. Interfaces 10, 13425–13433 (2018).
Hu, S. et al. Amorphous TiO2 coatings stabilize Si, GaAs, and GaP photoanodes for efficient water oxidation. Science 344, 1005–1009 (2014).
Yang, K., Kas, R. & Smith, W. A. In situ infrared spectroscopy reveals persistent alkalinity near electrode surfaces during CO2 electroreduction. J. Am. Chem. Soc. 141, 15891–15900 (2019).
Dinh, C.-T. et al. CO2 electroreduction to ethylene via hydroxide-mediated copper catalysis at an abrupt interface. Science 360, 783–787 (2018).
Wang, X. et al. Efficient electrically powered CO2-to-ethanol via suppression of deoxygenation. Nat. Energy 5, 478–486 (2020).
Kim, C. et al. Tailored catalyst microenvironments for CO2 electroreduction to multicarbon products on copper using bilayer ionomer coatings. Nat. Energy 6, 1026–1034 (2021).
Nesbitt, N. T. & Smith, W. A. Water and solute activities regulate CO2 reduction in gas-diffusion electrodes. J. Phys. Chem. C 125, 13085–13095 (2021).
Huang, J. E. et al. CO2 electrolysis to multicarbon products in strong acid. Science 372, 1074–1078 (2021).
Pelayo García de Arquer, F. et al. CO2 electrolysis to multicarbon products at activities greater than 1 A cm−2. Science 367, 661–666 (2020).
Gu, J. et al. Modulating electric field distribution by alkali cations for CO2 electroreduction in strongly acidic medium. Nat. Catal. 5, 268–276 (2022).
Shin, S. J. et al. A unifying mechanism for cation effect modulating C1 and C2 productions from CO2 electroreduction. Nat. Commun. 13, 5482 (2022).
Monteiro, M. C. O. et al. Absence of CO2 electroreduction on copper, gold and silver electrodes without metal cations in solution. Nat. Catal. 4, 654–662 (2021).
Goyal, A. & Koper, M. T. M. The interrelated effect of cations and electrolyte pH on the hydrogen evolution reaction on gold electrodes in alkaline media. Angew. Chem. Int. Ed. 60, 13452–13462 (2021).
Tang, B. Y., Bisbey, R. P., Lodaya, K. M., Toh, W. L. & Surendranath, Y. Reaction environment impacts charge transfer but not chemical reaction steps in hydrogen evolution catalysis. Nat. Catal. 6, 339–350 (2022).
Garcia, A. C., Touzalin, T., Nieuwland, C., Perini, N. & Koper, M. T. M. Enhancement of oxygen evolution activity of nickel oxyhydroxide by electrolyte alkali cations. Angew. Chem. 131, 13133–13137 (2019).
Luo, M. & Koper, M. T. M. A kinetic descriptor for the electrolyte effect on the oxygen reduction kinetics on Pt(111). Nat. Catal. 5, 615–623 (2022).
Fu, X. et al. Continuous-flow electrosynthesis of ammonia by nitrogen reduction and hydrogen oxidation. Science 379, 707–712 (2023).
Li, Y., Pei, Z., Luan, D. & Lou, X.-W. D. Superhydrophobic and conductive wire membrane for enhanced CO2 electroreduction to multicarbon products. Angew. Chem. Int. Ed. 62, e202302128 (2023).
Fu, Q. et al. Hybrid solar-to-methane conversion system with a Faradaic efficiency of up to 96%. Nano Energy 53, 232–239 (2018).
Pinaud, B. A. et al. Technical and economic feasibility of centralized facilities for solar hydrogen production via photocatalysis and photoelectrochemistry. Energy Environ. Sci. 6, 1983–2002 (2013).
Bolton, J. R., Strickler, S. J. & Connolly, J. S. Limiting and realizable efficiencies of solar photolysis of water. Nature 316, 495–500 (1985).
Nandy, S., Savant, S. A. & Haussener, S. Prospects and challenges in designing photocatalytic particle suspension reactors for solar fuel processing. Chem. Sci. 12, 9866–9884 (2021).
Goto, Y. et al. A particulate photocatalyst water-splitting panel for large-scale solar hydrogen generation. Joule 2, 509–520 (2018).
Boutin, E. et al. Photo-electrochemical conversion of CO2 under concentrated sunlight enables combination of high reaction rate and efficiency. Adv. Energy Mater. 12, 2200585 (2022).
Khan, B. et al. Unassisted photoelectrochemical CO2-to-liquid fuel splitting over 12% solar conversion efficiency. Nat. Commun. 15, 6990 (2024).
Zhang, Y., Yam, C. & Schatz, G. C. Fundamental limitations to plasmonic hot-carrier solar cells. J. Phys. Chem. Lett. 7, 1852–1858 (2016).
Ratchford, D. C., Dunkelberger, A. D., Vurgaftman, I., Owrutsky, J. C. & Pehrsson, P. E. Quantification of efficient plasmonic hot-electron injection in gold nanoparticle-TiO2 films. Nano Lett. 17, 6047–6055 (2017).
Chae, H. U. et al. High quantum efficiency hot electron electrochemistry. Nano Lett. 19, 6227–6234 (2019).
Govorov, A. O., Zhang, H. & Gun’Ko, Y. K. Theory of photoinjection of hot plasmonic carriers from metal nanostructures into semiconductors and surface molecules. J. Phys. Chem. C 117, 16616–16631 (2013).
Ye, W., Long, R., Huang, H. & Xiong, Y. Plasmonic nanostructures in solar energy conversion. J. Mater. Chem. C 5, 1008–1021 (2017).
Li, S. et al. Recent advances in plasmonic nanostructures for enhanced photocatalysis and electrocatalysis. Adv. Mater. 33, 2000086 (2021).
Mascaretti, L. & Naldoni, A. Hot electron and thermal effects in plasmonic photocatalysis. J. Appl. Phys. 128, 041101 (2020).
Zhang, C., Zhang, Y. & Xie, W. Plasmonic metal/semiconductor hybrid nanomaterials for solar to chemical energy conversion. J. Energy Chem. 63, 40–53 (2021).
Huang, H. et al. Solar-light-driven CO2 reduction by CH4 on silica-cluster-modified Ni nanocrystals with a high solar-to-fuel efficiency and excellent durability. Adv. Energy Mater. 8, 1702472 (2018).
Ng, C., Yap, L. W., Roberts, A., Cheng, W. & Gómez, D. E. Black gold: broadband, high absorption of visible light for photochemical systems. Adv. Funct. Mater. 27, 1604080 (2017).
Xi, S., Wang, L., Xie, H. & Yu, W. Solar-thermal energy conversion and storage of super black carbon reinforced melamine foam aerogel for shape-stable phase change composites. Int. J. Hydrog. Energy 47, 12024–12035 (2022).
Chueh, W. C. et al. High-flux solar-driven thermochemical dissociation of CO2 and H2O using nonstoichiometric ceria. Science 330, 1797–1801 (2010).
Romero, M. & Steinfeld, A. Concentrating solar thermal power and thermochemical fuels. Energy Environ. Sci. 5, 9234 (2012).
Marxer, D., Furler, P., Takacs, M. & Steinfeld, A. Solar thermochemical splitting of CO2 into separate streams of CO and O2 with high selectivity, stability, conversion, and efficiency. Energy Environ. Sci. 10, 1142–1149 (2017).
Vos de, A. & Pauwels, H. On the thermodynamic limit of photovoltaic energy conversion. Appl. Phys. 25, 119–125 (1981).
King, R. R. et al. Solar cell generations over 40% efficiency. Prog. Photovolt. Res. Appl. 20, 801–815 (2012).
Wang, P. et al. Numerical study of mono-crystalline silicon solar cells with passivated emitter and rear contact configuration for the efficiency beyond 24% based on mass production technology. J. Semicond. 41, 062701 (2020).
Oni, A. M., Mohsin, A. S. M., Rahman, M. & Hossain, M. B. B. A comprehensive evaluation of solar cell technologies, associated loss mechanisms, and efficiency enhancement strategies for photovoltaic cells. Energy Rep. 11, 3345–3366 (2024).
Burrin, D., Roy, S., Roskilly, A. P. & Smallbone, A. A combined heat and green hydrogen (CHH) generator integrated with a heat network. Energy Convers. Manag. 246, 114686 (2021).
María Villarreal Vives, A., Wang, R., Roy, S. & Smallbone, A. Techno-economic analysis of large-scale green hydrogen production and storage. Appl. Energy 346, 121333 (2023).
Johnson, E. F. & Haussener, S. Thermal-integration in photoelectrochemistry for fuel and heat co-generation. Sustain. Energy Fuels 8, 4199–4212 (2024).
Yuan, Y. et al. Earth-abundant photocatalyst for H2 generation from NH3 with light-emitting diode illumination. Science 893, 889–893 (2022).
Mohan, A. et al. Hybrid photo-and thermal catalyst system for continuous CO2 reduction. ACS Appl. Mater. Interfaces 12, 33613–33620 (2020).
Andrei, V. et al. Floating perovskite-BiVO4 devices for scalable solar fuel production. Nature 608, 518–522 (2022).
Hansora, D. et al. All-perovskite-based unassisted photoelectrochemical water splitting system for efficient, stable and scalable solar hydrogen production. Nat. Energy. 9, 272–284 (2024).
Solhyd project team. The Solhyd project is launched! Solhyd https://solhyd.eu/en/solhyd_blog_en/the-solhyd-project-launched (2021).
Solhyd. Solhyd, Nippon Gases, Ether Energy and SunBuild to build world’s first solar hydrogen park, setting a new standard in hydrogen technology. Solhyd https://solhyd.eu/en/solhyd_blog_en/solhyd-nippon-gases-ether-energy-and-sunbuild-to-build-worlds-first-solar-hydrogen-park-setting-a-new-standard-in-hydrogen-technology (2025).
Naimovičius, L., Bharmoria, P. & Moth-Poulsen, K. Triplet–triplet annihilation mediated photon upconversion solar energy systems. Mater. Chem. Front. 7, 2297 (2023).
Li, X. et al. Spectral response regulation strategy by downshifting materials to improve efficiency of flexible perovskite solar cells. Nano Energy 114, 108619 (2023).
Meinardi, F., Bruni, F. & Brovelli, S. Luminescent solar concentrators for building-integrated photovoltaics. Nat. Rev. Mater. 2, 17072 (2017).
Belsa, B. et al. Materials challenges on the path to gigatonne CO2 electrolysis. Nat. Rev. Mater. 9, 535–549 (2024).
Fu, H. C. et al. Improved performance and stability of photoelectrochemical water-splitting Si system using a bifacial design to decouple light harvesting and electrocatalysis. Nano Energy 70, 104478 (2020).
Dihan, M. R. et al. Photocatalytic and photoelectrochemical H2 generation for sustainable future: performance improvement, techno-economic analysis, and life cycle assessment for shaping the reality. Fuel 392, 134356 (2025).
Koshy, D. M. et al. Bridging thermal catalysis and electrocatalysis: catalyzing CO2 conversion with carbon-based materials. Angew. Chem. Int. Ed 60, 17472–17480 (2021).
Pornrungroj, C. et al. Hybrid photothermal–photocatalyst sheets for solar-driven overall water splitting coupled to water purification. Nat. Water 1, 952–960 (2023).
Wang, Q. & Domen, K. Particulate photocatalysts for light-driven water splitting: mechanisms, challenges, and design strategies. Chem. Rev. 120, 919–985 (2020).
Xiao, Z. et al. A comprehensive review on photo-thermal co-catalytic reduction of CO2 to value-added chemicals. Fuel 362, 130906 (2024).
Wu, S., Wu, S. & Sun, Y. Light-driven dry reforming of methane on metal catalysts. Sol. RRL 5, 2000507 (2021).
Haley, J., Leach, C., Jordan, B., Dehoff, R. & Paquit, V. In-situ digital image correlation and thermal monitoring in directed energy deposition additive manufacturing. Opt. Express 29, 9927–9941 (2021).
Sastre, F. et al. Sunlight-fueled, low-temperature Ru-catalyzed conversion of CO2 and H2 to CH4 with a high photon-to-methane efficiency. ACS Omega 4, 7369–7377 (2019).
Cortés, E. et al. Challenges in plasmonic catalysis. ACS Nano 14, 16202–16219 (2020).
Elias, R. C., Yan, B. & Linic, S. Probing spatial energy flow in plasmonic catalysts from charge excitation to heating: nonhomogeneous energy distribution as a fundamental feature of plasmonic chemistry. J. Am. Chem. Soc. 146, 29656–29663 (2024).
Qi, Y. et al. Unraveling of cocatalysts photodeposited selectively on facets of BiVO4 to boost solar water splitting. Nat. Commun. 13, 484 (2022).
Cobb, S. J. et al. A photoelectrochemical-thermoelectric device for semi-artificial CO2 fixation employing full solar spectrum utilization. Device 2, 100505 (2024).
Zhou, S. et al. Nanoengineered kesterite photocathodes: enhancing photoelectrochemical performance for water splitting and beyond. ACS Nano 19, 17041–17061 (2025).
Ni, J. et al. Deciphering electrolyte selection for electrochemical reduction of carbon dioxide and nitrogen to high-value-added chemicals. Adv. Funct. Mater. 33, 2212483 (2023).
Pornrungroj, C. et al. Bifunctional perovskite-BiVO4 tandem devices for uninterrupted solar and electrocatalytic water splitting cycles. Adv. Funct. Mater. 31, 1–9 (2021).
Ampelli, C. et al. An artificial leaf device built with earth-abundant materials for combined H2 production and storage as formate with efficiency > 10%. Energy Environ. Sci. 16, 1644–1661 (2023).
Sivula, K. Surface modification of semiconductor photoelectrodes. Phys. Chem. Chem. Phys. 17, 15655–15674 (2015).
Arinze, E. S., Qiu, B., Nyirjesy, G. & Thon, S. M. Plasmonic nanoparticle enhancement of solution-processed solar cells: practical limits and opportunities. ACS Photon. 3, 158–173 (2016).
Christopher, P., Xin, H. & Linic, S. Visible-light-enhanced catalytic oxidation reactions on plasmonic silver nanostructures. Nat. Chem. 3, 467–472 (2011).
Kang, Y. et al. An integrated thermoelectric-assisted photoelectrochemical system to boost water splitting. Sci. Bull. 65, 1163–1169 (2020).
Tembhurne, S., Nandjou, F. & Haussener, S. A thermally synergistic photo-electrochemical hydrogen generator operating under concentrated solar irradiation. Nat. Energy 4, 399–407 (2019).
Pornrungroj, C., Andrei, V. & Reisner, E. Thermoelectric−photoelectrochemical water splitting under concentrated solar irradiation. J. Am. Chem. Soc. 145, 13709–13714 (2023).
Schneidewind, J. How much technological progress is needed to make solar hydrogen cost-competitive? Adv. Energy Mater. 12, 2200342 (2022).
Ding, X., Liu, W., Zhao, J., Wang, L. & Zou, Z. Photothermal CO2 catalysis toward the synthesis of solar fuel: from material and reactor engineering to techno-economic analysis. Adv. Mater. 37, 1–15 (2025).
Kurup, P., Glynn, S. & Akar, S. Manufacturing cost analysis of advanced parabolic trough collector. AIP Conf. Proc. 2445, 020006 (2022).
Hong, J. et al. Photothermal chemistry based on solar energy: from synergistic effects to practical applications. Adv. Sci. 9, e2103926 (2022).
Chen, P., Zhang, Y., Zhou, Y. & Dong, F. Photoelectrocatalytic carbon dioxide reduction: fundamental, advances and challenges. Nano Mater. Sci. 3, 344–367 (2021).
Fu, Y., Wang, Y., Huang, J., Lu, K. & Liu, M. Solar fuel production through concentrating light irradiation. Green Energy Environ. 9, 1550–1580 (2024).
He, J. & Janáky, C. Recent advances in solar-driven carbon dioxide conversion: expectations versus reality. ACS Energy Lett. 5, 1996–2014 (2020).
Galushchinskiy, A., González-Gómez, R., McCarthy, K., Farràs, P. & Savateev, A. Progress in development of photocatalytic processes for synthesis of fuels and organic compounds under outdoor solar light. Energy Fuels 36, 4625–4639 (2022).
Syzygy Plasmonics. Illuminating the future of chemical manufacturing. Syzygy Plasmonics https://syzygyplasmonics.squarespace.com (2025).
Luterbacher, C. A solar hydrogen system that co-generates heat and oxygen. EPFL https://actu.epfl.ch/news/a-solar-hydrogen-system-that-co-generates-heat-and (2023).
Shaner, M. R., Atwater, H. A., Lewis, N. S. & McFarland, E. W. A comparative technoeconomic analysis of renewable hydrogen production using solar energy. Energy Environ. Sci. 9, 2354–2371 (2016).
Acknowledgements
ICFO thanks CEX2024-001490-S and PID2022-138127NA-I00 (MCIN/AEI/10.13039/501100011033), Fundació Cellex, Fundació Mir-Puig, BIST Ignite (7th edition), Generalitat de Catalunya through CERCA (SGR 2021 01455); Fundación Ramón Areces (CIVP21S13318); the European Union: NASCENT (101077243) and ICONIC (101115204). Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the European Union or the European Innovation Council and SMEs Executive Agency (EISMEA). Neither the European Union nor the granting authority can be held responsible for them.
Author information
Authors and Affiliations
Contributions
All authors contributed to the research, discussion and editing. All authors reviewed the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Clean Technology thanks Peng Chen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Golovanova, V., Mittal, D. & García de Arquer, F.P. What solar fuel technologies can learn from each other. Nat. Rev. Clean Technol. (2026). https://doi.org/10.1038/s44359-025-00130-5
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s44359-025-00130-5