Abstract
Study design:
Female Wistar rats (225 g) underwent spinal cord injury (SCI) at the T4 segment and were assigned to one of the three groups treated with: (1) saline; (2) 7.5 mg kg–1 Reparixin; or (3) 15 mg kg–1 Reparixin. Reparixin is a small molecule, allosteric noncompetitive inhibitor of CXCR1 and CXCR2 chemokine receptors involved in inflammation.
Methods:
Spinal cord homogenates at 12 and 72 h post-SCI were assayed for tumor necrosis factor α (TNF-α) and cytokine-induced neutrophil chemoattractant (CINC)-1 using enzyme-linked immunosorbant assay (ELISA). Myeloperoxidase activity and western blots for CD68, Fas and p75 content were used to assess inflammation and death receptor ligands, respectively. Histopathology and neurological outcomes were assessed by immunohistochemistry, locomotion scoring and cardiovascular measurement of autonomic dysreflexia 4 weeks post-SCI.
Results:
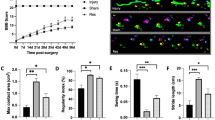
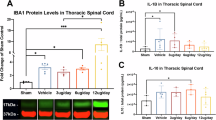
Both 7.5 and 15 mg kg–1 doses of Reparixin reduced levels of TNF-α and CINC-1 72 h post-SCI and decreased macrophage (CD68) content in the spinal cord lesion. Only 15 mg kg–1 Reparixin reduced both Fas and p75 levels in the spinal cord compared with untreated SCI. We observed a reduced lesion area and increased neuron number in the gray matter of Reparixin-treated rats. Hindlimb motor scores at 7 and 28 days post-SCI were improved by 15 mg kg–1 Reparixin treatment. Both 7.5 and 15 mg kg–1 Reparixin reduced development of autonomic dysreflexia 4 weeks post-SCI. The change in mean arterial pressure, induced by cutaneous or visceral stimulation, was reduced by 40–50%.
Conclusion:
Acute treatment with 15 mg kg–1 Reparixin reduces acute inflammation and is associated with minor improvements in motor function and a significant reduction in the severity of autonomic dysreflexia.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Kwon BK, Tetzlaff W, Grauer JN, Beiner J, Vaccaro AR . Pathophysiology and pharmacologic treatment of acute spinal cord injury. Spine J 2004; 4: 451–464.
Gris D, Marsh DR, Oatway MA, Chen Y, Hamilton EF, Dekaban GA et al. Transient blockade of the CD11d/CD18 integrin reduces secondary damage after spinal cord injury, improving sensory, autonomic, and motor function. J Neurosci 2004; 24: 4043–4051.
Gonzalez R, Glaser J, Liu MT, Lane TE, Keirstead HS . Reducing inflammation decreases secondary degeneration and functional deficit after spinal cord injury. Exp Neurol 2003; 184: 456–463.
Ghirnikar RS, Lee YL, Eng LF . Chemokine antagonist infusion promotes axonal sparing after spinal cord contusion injury in rat. J Neurosci Res 2001; 64: 582–589.
Ackery A, Robins S, Fehlings MG . Inhibition of Fas-mediated apoptosis through administration of soluble Fas receptor improves functional outcome and reduces posttraumatic axonal degeneration after acute spinal cord injury. J Neurotrauma 2006; 23: 604–616.
Demjen D, Klussmann S, Kleber S, Zuliani C, Stieltjes B, Metzger C et al. Neutralization of CD95 ligand promotes regeneration and functional recovery after spinal cord injury. Nat Med 2004; 10: 389–395.
Gorio A, Madaschi L, Zadra G, Marfia G, Cavalieri B, Bertini R et al. Reparixin, an inhibitor of CXCR2 function, attenuates inflammatory responses and promotes recovery of function after traumatic lesion to the spinal cord. J Pharmacol Exp Ther 2007; 322: 973–981.
Gris D, Marsh DR, Dekaban GA, Weaver LC . Comparison of effects of methylprednisolone and anti-CD11d antibody treatments on autonomic dysreflexia after spinal cord injury. Exp Neurol 2005; 194: 541–549.
Valles A, Grijpink-Ongering L, de Bree FM, Tuinstra T, Ronken E . Differential regulation of the CXCR2 chemokine network in rat brain trauma: implications for neuroimmune interactions and neuronal survival. Neurobiol Dis 2006; 22: 312–322.
Bertini R, Allegretti M, Bizzarri C, Moriconi A, Locati M, Zampella G et al. Noncompetitive allosteric inhibitors of the inflammatory chemokine receptors CXCR1 and CXCR2: prevention of reperfusion injury. Proc Natl Acad Sci USA 2004; 101: 11791–11796.
Weaver LC, Verghese P, Bruce JC, Fehlings MG, Krenz NR, Marsh DR . Autonomic dysreflexia and primary afferent sprouting after clip-compression injury of the rat spinal cord. J Neurotrauma 2001; 18: 1107–1119.
Lindan R, Joiner E, Freehafer AA, Hazel C . Incidence and clinical features of autonomic dysreflexia in patients with spinal cord injury. Parap 1980; 18: 285–292.
Karlsson A-K . Autononomic dysreflexia. Spinal Cord 1999; 37: 383–391.
Eltorai I, Kim R, Vulpe M, Kasravi H, Ho W . Fatal cerebral hemorrhage due to autonomic dysreflexia in a tetraplegic patient: case report and review. Parap 1992; 30: 355–360.
Casha S, Yu WR, Fehlings MG . Oligodendroglial apoptosis occurs along degenerating axons and is associated with FAS and P75 expression following spinal cord injury. Neurosci 2001; 103: 203–218.
Eikard B, Andersen JR . Arrhythmias during halothane anaesthesia II: the influence of atropine. Acta Anaesthesiol Scand 1977; 21: 245–251.
Marsh DR, Weaver LC . Autonomic dysreflexia, induced by noxious or innocuous stimulation, does not depend on changes in dorsal horn substance p. J Neurotrauma 2004; 21: 817–828.
Bao F, Chen Y, Dekaban GA, Weaver LC . Early anti-inflammatory treatment reduces lipid peroxidation and protein nitration after spinal cord injury in rats. J Neurochem 2004; 88: 1335–1344.
Basso DM, Beattie MS, Bresnahan JC . A sensitive and reliable locomotor rating scale for open field testing in rats. J.Neurotrauma 1995; 12: 1–21.
Ditor DS, John S, Cakiroglu J, Kittmer C, Foster PJ, Weaver LC . Magnetic resonance imaging versus histological assessment for estimation of lesion volume after experimental spinal cord injury Laboratory investigation. J Neurosurg Spine 2008; 9: 301–306.
Acknowledgements
This research was funded by the Nova Scotia Heart and Stroke Foundation in a grant to DM. In addition, JF was supported by a research fellowship from the Dalhousie Faculty of Medicine and with funding from Dompé Pharma spa., L’Aquila, Italy. We thank Ricardo Bertini at Dompé Pharma spa. for providing Reparixin for the project and valuable information regarding its appropriate in vivo administration. We also thank Ahmed Ghaly for preparing saline and drug treatments and keeping the code private until results were complete
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Marsh, D., Flemming, J. Inhibition of CXCR1 and CXCR2 chemokine receptors attenuates acute inflammation, preserves gray matter and diminishes autonomic dysreflexia after spinal cord injury. Spinal Cord 49, 337–344 (2011). https://doi.org/10.1038/sc.2010.127
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sc.2010.127
Keywords
This article is cited by
-
MHC/class-II-positive cells inhibit corticosterone of adrenal gland cells in experimental arthritis: a role for IL-1β, IL-18, and the inflammasome
Scientific Reports (2020)
-
Selection and Prioritization of Candidate Drug Targets for Amyotrophic Lateral Sclerosis Through a Meta-Analysis Approach
Journal of Molecular Neuroscience (2017)
-
Multiple organ dysfunction and systemic inflammation after spinal cord injury: a complex relationship
Journal of Neuroinflammation (2016)