Abstract
Study design:
Cervical spondylotic myelopathy (CSM) represents the most commonly acquired cause of spinal cord dysfunction among individuals over 55 years old. The pathophysiology of the disease involves static and dynamic mechanical factors, which are the result of chronic degeneration. The clinical course of the disease remains unpredictable. In the past, many experimental animal models have been developed to study the cellular and molecular mechanisms underlining the pathophysiology of the disease.
Objectives:
To create a new animal model of CSM, which will reproduce the temporal course of the disease and the local microenvironment at the site of spinal cord compression.
Methods:
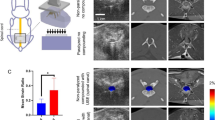
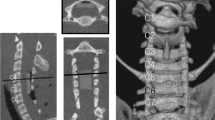
We performed posterior laminectomy to New Zealand rabbits at the level of C7, and a thin sheet (5–7 μm) of aromatic polyether was implanted with microsurgical technique at the epidural space underneath C5–C6 laminae. Motor function evaluation was performed after the operation and once a week thereafter.
Results:
After 20 weeks, the animals were killed, and the histological evaluation of spinal cord at the site of compression above and below it, using eosin hematoxylin, immonohistochemistry and Kluver–Barrera techniques reveals axonal swelling and demyelination, interstitial edema and myelin sheet fragmentation. Moreover, histological evaluation of C5 and C6 laminae reveals osteophyte formation.
Conclusion:
We believe that this CSM model reproduces the temporal evolution of the disease and creates a local microenvironment at the site of spinal cord compression, which shares the same characteristics with that of human disease.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Imai T . Cervical spondylotic myelopathy and sagittal diameter of the cervical canal]. Nippon Seikeigeka Gakkai Zasshi 1970; 44: 429–438.
White III AA, Panjabi MM . Biomechanical considerations in the surgical management of cervical spondylotic myelopathy. Spine 1988; 13: 856–860.
Robinson RA, Afeiche N, Dunn EJ, Northrup BE . Cervical spondylotic myelopathy: etiology and treatment concepts. Spine 1977; 2: 89–99.
Baptiste DC, Fehlings MG . Pathophysiology of cervical myelopathy. Spine J 2006; 6: 190S–197S.
Barron EM, Young WF . Cervical spondylotic myelopathy: a brief review of its pathophysiology clinical course, and diagnosis. Neurosurgery 2007; 60 (1 Supp1 1): S35–S41.
Barnes MP, Saunders M . The effect of cervical mobility on the natural history of cervical spondylotic myelopathy. J Neurol Neurosurg Psychiatry 1984; 47: 17–20.
Hogan El, Romanul FCA . Spinal cord infarction occurring during insertion of aortic graft. Neurology 1966; 16: 67–74.
Mair WGP, Druckman R . The pathology of spinal cord lesions and their relation to the clinical features in protrusion of cervical intervertebral discs (a report of four cases). Brain 1953; 76: 70–91.
Karadimas SK, Gialeli CH, Klironomos G, Tzanakakis GN, Panagiotopoulos E, Karamanos NK et al. The role of oligodendrocytes in the molecular pathobiology and potential molecular treatment of cervical spondylotic myelopathy. Curr Med Chem 2010; 17: 1048–1058.
Tyllianakis M, Dalas E, Christofidou M, Kallitsis JK, Chrissanthopoulos A, Koutsoukos PG et al. Novel composites materials from functionalized polymers and silver coated titanium oxide capable for calcium phosphate induction, control of orthopedic biofilm infections: an ‘in vitro’ study. J Mater Sci Mater Med 2010; 21: 2201–2211.
Ito T, Oyanagi K, Takahashi H, Takahashi HE, Ikuta F . Cervical spondylosis myelopathy: clinicopathologic study on the progression pattern and thin myelinated fibers of the lesions of seven patients examined during complete autopsy. Spine 1996; 21: 827–833.
Ogino H, Tada K, Okada K, Yonenobu K, Yamamoto T, Ono K et al. Canal diameter, antero-posterior compression ratio, and spondylotic myelopathy of the cervical spine. Spine 1983; 8: 1–15.
Payne LW, Spillane JD . The cervical spine, an anatomical study of 70 specimens, with particular reference to the problem of cervical spondylosis. Brain 1957; 80: 571–596.
Fehlings MG, Yu WR, Shannon P, Sekhon LHS . Molecular mechanisms of cell death in human cervical spondylotic myelopathy: evidence for apoptosis, death receptor expression and Caspase 3 activation. Proceedings of the 28th Annual Meeting of the Cervical Spine Research Society 2000.
Silver J, Miller JH . Regeneration beyond the glial scar. Nat Rev Neurosci 2004; 5: 146–156.
Ridet JL, Malhotra SK, Privat A, Gage FH . Reactive astrocytes: cellular and molecular cues to biological function. Trends Neurosci 1997; 20: 570–577.
Fitch MT, Doller C, Combs CK, Landreth GE, Silver J . Cellular and molecular mechanisms of glial scarring and progressive cavitation: in vivo and in vitro analysis of inflammation-induced secondary injury after CNS trauma. J Neurosci 1999; 19: 8182–8198.
McGraw J, Hiebert GW, Steeves JD . Modulating astrogliosis after neurotrauma. J Neurosci Res 2001; 63: 109–115.
Matyash M, Matyash V, Nolte C, Sorrentino V, Kettenmann H . Requirement of functional ryanodine receptor type 3 for astrocyte migration. FASEB J 2002; 16: 84–86.
Kim P, Haisa T, Kawamoto T, Kirino T, Wakai S . Delayed myelopathy induced by chronic compression in the rat spinal cord. Ann Neurol 2004; 55: 503–511.
Kanchiku T, Taguchi T, Kaneko K, Yonemura H, Kawai S, Gondo T . A new rabbit model for the study on cervical compressive myelopathy. J Orthop Res 2001; 19: 605–613.
Acknowledgements
We thank Professor JK Kallitsis of Advanced Polymers & Hybrid Nanomaterials Research Lab, Department of Chemistry, University of Patras, Rio-Patras, Greece.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Klironomos, G., Karadimas, S., Mavrakis, A. et al. New experimental rabbit animal model for cervical spondylotic myelopathy. Spinal Cord 49, 1097–1102 (2011). https://doi.org/10.1038/sc.2011.71
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sc.2011.71
Keywords
This article is cited by
-
Degenerative cervical myelopathy — update and future directions
Nature Reviews Neurology (2020)
-
Cervical excitatory neurons sustain breathing after spinal cord injury
Nature (2018)
-
Pathobiology of cervical spondylotic myelopathy
European Spine Journal (2015)
-
Arterial arrangement of the cervical spinal cord in rabbit
Anatomical Science International (2012)