Abstract
Study design:
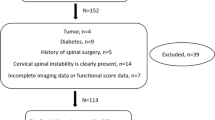
A single-center magnetic resonance imaging and spectroscopic study involving 21 patients with advanced cervical spondylosis and 11 healthy controls.
Objective:
We assessed the utility of magnetic resonance spectroscopy (MRS) to quantify biochemical changes within the spinal cord and serve as a potential biomarker in patients with cervical spondylosis with or without T2 hyperintensity within the cord.
Setting:
Los Angeles, California, USA.
Methods:
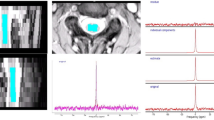
Twenty-one patients with cervical spondylosis and eleven healthy controls were evaluated. Single-voxel MRS was performed in the cervical cord. Morphometry of the spinal canal space was measured. N-Acetyl aspartylglutamic acid (NAA), choline (Cho), myo-inositol (Myo-I), glutamine–glutamate complex (Glx) and lactate metabolite concentration ratios with respect to total creatine (Cr) were quantified using an LC model algorithm and compared between healthy controls and spondylosis patients. Correlation of MRS metabolites with modified Japanese Orthopaedic Association (mJOA) score was also performed.
Results:
The spinal canal space was significantly different between patients and controls (analysis of variance (ANOVA), P<0.0001). Total Cho-to-Cr ratio was significantly elevated in patients with spondylosis and T2-hyperintensity compared with healthy controls (ANOVA, P<0.01). A significantly higher Cho-to-NAA ratio was observed in spondylosis patients compared with healthy controls (ANOVA, P<0.01). Slightly elevated Glx and Myo-I were encountered in patients with stenosis without T2 hyperintensity. A linear correlation between Cho-NAA ratio and mJOA was also observed (P<0.01).
Conclusion:
MRS appears sensitive to biochemical changes occurring in advanced cervical spondylosis patients. The Cho/NAA ratio was significantly correlated with the mJOA score, providing a potentially clinically useful radiographical biomarker for the management of advanced cervical spondylosis patients.
Sponsorship:
NIH NINDS 1R21NS65419-1A1; NIH/NINDS 1R01NS078494-01A1.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Moore AP, Blumhardt LD . A prospective survey of the causes of non-traumatic spastic paraparesis and tetraparesis in 585 patients. Spinal Cord 1997; 35: 361–367.
Young WF . Cervical spondylotic myelopathy: a common cause of spinal cord dysfunction in older persons. Am Fam Physician 2000; 62: 1064–1070.
Wang MC, Chan L, Maiman DJ, Kreuter W, Deyo RA . Complications and mortality associated with cervical spine surgery for degenerative disease in the United States. Spine 2007; 32: 342–347.
Sampath P, Bendebba M, Davis JD, Ducker TB . Outcome of patients treated for cervical myelopathy. A prospective, multicenter study with independent clinical review. Spine 2000; 25: 670–676.
Irwin ZN, Hilibrand A, Gustavel M, McLain R, Shaffer W, Myers M et al. Variation in surgical decision making for degenerative spinal disorders. Part II: cervical spine. Spine 2005; 30: 2214–2219.
Harkey HL, al-Mefty O, Marawi I, Peeler DF, Haines DE, Alexander LF . Experimental chronic compressive cervical myelopathy: effects of decompression. J Neurosurg 1995; 83: 336–341.
Fernandez de Rota JJ, Meschian S, Fernandez de Rota A, Urbano V, Baron M . Cervical spondylotic myelopathy due to chronic compression: the role of signal intensity changes in magnetic resonance images. J Neurosurg Spine 2007; 6: 17–22.
Yukawa Y, Kato F, Ito K, Horie Y, Hida T, Machino M et al. Postoperative changes in spinal cord signal intensity in patients with cervical compression myelopathy: comparison between preoperative and postoperative magnetic resonance images. J Neurosurg Spine 2008; 8: 524–528.
Yukawa Y, Kato F, Yoshihara H, Yanase M, Ito K . MR T2 image classification in cervical compression myelopathy: predictor of surgical outcomes. Spine 2007; 32: 1675–1678 discussion 1679.
Holly LT, Freitas B, McArthur DL, Salamon N . Proton magnetic resonance spectroscopy to evaluate spinal cord axonal injury in cervical spondylotic myelopathy. J Neurosurg Spine 2009; 10: 194–200.
Yonenobu K, Abumi K, Nagata K, Taketomi E, Ueyama K . Interobserver and intraobserver reliability of the japanese orthopaedic association scoring system for evaluation of cervical compression myelopathy. Spine (Phila Pa 1976) 2001; 26: 1890–1894 discussion 1895.
Benzel EC, Lancon J, Kesterson L, Hadden T . Cervical laminectomy and dentate ligament section for cervical spondylotic myelopathy. J Spinal Disord 1991; 4: 286–295.
Provencher SW . Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 1993; 30: 672–679.
Provencher SW . Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed 2001; 14: 260–264.
Tierney RT, Maldjian C, Mattacola CG, Straub SJ, Sitler MR . Cervical Spine Stenosis Measures in Normal Subjects. J Athl Train 2002; 37: 190–193.
Torg JS, Corcoran TA, Thibault LE, Pavlov H, Sennett BJ, Naranja RJ Jr et al. Cervical cord neurapraxia: classification, pathomechanics, morbidity, and management guidelines. J Neurosurg 1997; 87: 843–850.
Herzog RJ, Wiens JJ, Dillingham MF, Sontag MJ . Normal cervical spine morphometry and cervical spinal stenosis in asymptomatic professional football players. Plain film radiography, multiplanar computed tomography, and magnetic resonance imaging. Spine (Phila Pa 1976) 1991; 16 (6 Suppl): S178–S186.
Elliott JM, Pedler AR, Cowin G, Sterling M, McMahon K . Spinal cord metabolism and muscle water diffusion in whiplash. Spinal Cord 2011; 50: 474–476.
Ciccarelli O, Wheeler-Kingshott CA, McLean MA, Cercignani M, Wimpey K, Miller DH et al. Spinal cord spectroscopy and diffusion-based tractography to assess acute disability in multiple sclerosis. Brain 2007; 130 (Pt 8): 2220–2231.
Marliani AF, Clementi V, Albini Riccioli L, Agati R, Carpenzano M, Salvi F et al. Quantitative cervical spinal cord 3T proton MR spectroscopy in multiple sclerosis. AJNR Am J Neuroradiol 2010; 31: 180–184.
Verma P, Garcia-Alias G, Fawcett JW . Spinal cord repair: bridging the divide. Neurorehabil Neural Repair 2008; 22: 429–437.
Erschbamer M, Oberg J, Westman E, Sitnikov R, Olson L, Spenger C . 1H-MRS in spinal cord injury: acute and chronic metabolite alterations in rat brain and lumbar spinal cord. Eur J Neurosci 2011; 33: 678–688.
Ashwal S, Holshouser B, Tong K, Serna T, Osterdock R, Gross M et al. Proton MR spectroscopy detected glutamate/glutamine is increased in children with traumatic brain injury. J Neurotrauma 2004; 21: 1539–1552.
Blamire AM, Cader S, Lee M, Palace J, Matthews PM . Axonal damage in the spinal cord of multiple sclerosis patients detected by magnetic resonance spectroscopy. Magn Reson Med 2007; 58: 880–885.
Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AM . N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog Neurobiol 2007; 81: 89–131.
Sajja BR, Wolinsky JS, Narayana PA . Proton magnetic resonance spectroscopy in multiple sclerosis. Neuroimaging Clin N Am 2009; 19: 45–58.
Ellingson BM, Schmit BD, Kurpad SN . Lesion growth and degeneration patterns measured using diffusion tensor 9.4-T magnetic resonance imaging in rat spinal cord injury. J Neurosurg Spine 2010; 13: 181–192.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Salamon, N., Ellingson, B., Nagarajan, R. et al. Proton magnetic resonance spectroscopy of human cervical spondylosis at 3T. Spinal Cord 51, 558–563 (2013). https://doi.org/10.1038/sc.2013.31
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sc.2013.31
Keywords
This article is cited by
-
Evaluating tissue injury in cervical spondylotic myelopathy with spinal cord MRI: a systematic review
European Spine Journal (2024)
-
Degenerative cervical myelopathy: Where have we been? Where are we now? Where are we going?
Acta Neurochirurgica (2023)