Abstract
Study design:
Experimental study.
Objectives:
To investigate the effect of pre-treatment with PMX53, a C5aR antagonist, on spinal cord ischemia-reperfusion injury (IRI) in rat.
Setting:
Department of Neurosurgery, Second Affiliated Hospital, Xi’an Jiaotong University School of Medicine, Xi’an, Shaanxi Province, China.
Methods:
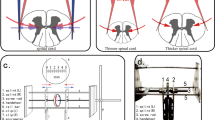
IRI was induced in the lumbar spinal cord by applying a mini aneurysm clamp to the abdominal aorta for 60 min in adult Sprague–Dawley rats. PMX53 (1 mg kg−1) was administered through femoral vein injection 30 min before ischemia on the rats in the PMX53 group (n=18). The saline group (n=18) was given saline at the same volume through femoral vein injection. The neurologic outcome of the posterior limbs was assessed by the Basso-Beattie-Bresnahan (BBB) score at 1, 6, 12, 24 and 48 h after reperfusion. Histologic changes of the spinal cord were detected with hematoxylin–eosin (H–E) staining. Enzyme-linked immunosorbent assay (ELISA) was used to detect myeloperoxidase (MPO) activity in the spinal cord. Immunohistochemistry was used to investigate the quantity of activated astrocytes and microglia.
Results:
After pre-treatment with PMX53, neurologic function improved gradually after 6, 12, 24 and 48 h reperfusion. The BBB score of the PMX53 group increased significantly (P<0.05) compared with the saline group. H–E staining showed that pathologic damage in the PMX53 group was reduced. Moreover, administration of PMX53 significantly inhibited neutrophil infiltration in the spinal cord. Levels of MPO activity in the spinal cord were remarkably lower in the PMX53 group (P<0.05). There were also more activated microglia and astrocytes in the spinal cord of the PMX53 group than in the saline group (P<0.05).
Conclusion:
PMX53 delivered 30 min prior to ischemic injury protects the spinal cord from IRI, probably via the inhibition of neutrophil activity, increased activated microglia and astrocyte.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Kanellopoulos GK, Kato H, Hsu CY, Kouchoukos NT . Spinal cord ischemic injury. Development of a new model in the rat. Stroke 1997; 28: 2532–2538.
Carmona P, Mateo E, Otero M, Marques JI, Pena JJ, Llagunes J et al. Spinal cord protection during open and endovascular surgery in thoracic and thoracoabdominal aorta diseases. Rev Esp Anestesiol Reanim 2011; 58: 110–118.
Lintott P, Hafez HM, Stansby G . Spinal cord complications of thoracoabdominal aneurysm surgery. Br J Surg 1998; 85: 5–15.
Wan IY, Angelini GD, Bryan AJ, Ryder I, Underwood MJ . Prevention of spinal cord ischemia during descending thoracoabdominal aortic surgery. Eur J Cardiothorac Surg 2001; 19: 203–213.
Hasturk A, Atalay B, Calisaneller T, Ozdemir O, Oruckaptan H, Altinors N . Analysis of serum pro-inflammatory cytokine levels after rat spinal cord ischemia/reperfusion injury and correlation with tissue damage. Turk Neurosurg 2009; 19: 353–359.
Maureen M . Ischemia–reperfusion injury: assessment and treatment, part II. J Vet Emerg Crit Care 2004; 14: 242–252.
Lewen A, Matz P, Chan PH . Free radical pathways in CNS injury. J Neurotrauma 2000; 17: 871–890.
Marsala M, Sorkin LS, Yaksh TL . Transient spinal ischemia in rat: characterization of spinal cord blood flow, extracellular amino acid release, and concurrent histopathological damage. J Cereb Blood Flow Metab 1994; 14: 604–614.
Gong S, Peng L, Yan B, Dong Q, Seng Z, Wang W et al. Bosentan reduces neuronal apoptosis following spinal cord ischemic reperfusion injury. Spinal cord 2013; 52: 181–185.
Gong S, Seng Z, Wang W, Lv J, Dong Q, Yan B et al. Bosentan protects the spinal cord from ischemia reperfusion injury in rats through vascular endothelial growth factor receptors [J]. Spinal cord 2014; 2014: 147.
Zhu P, Li J, Fujino M, Zhuang J, Li XK . Development and treatments of Inflammatory cells and cytokines in spinal cord ischemia-reperfusion injury. Mediators Inflamm 2013; 2013: 701970.
Benard M, Raoult E, Vaudry D, Leprince J, Falluel-Morel A, Gonzalez BJ et al. Role of complement anaphylatoxin receptors (C3aR, C5aR) in the development of the rat cerebellum. Mol Immunol 2008; 45: 3767–3774.
Stevens B, Allen NJ, Vazquez LE, Howell GR, Christopherson KS, Nouri N et al. The classical complement cascade mediates CNS synapse elimination. Cell 2007; 13: 1164–1178.
Rahpeymai Y, Hietala MA, Wilhelmsson U, Fotheringham A, Davies I, Nilsson AK et al. Complement: a novel factor in basal and ischemia-induced neurogenesis. EMBO J 2006; 25: 1364–1374.
Dersen ED, Loberg EM, Vege E, Daha MR, Maehlen J, Mollnes TE . In situ deposition of complement in human acute brain ischaemia. Scand J Immunol 2009; 69: 555–562.
Anderson A, Robert S, Huang W, Young W, Cotman CW . Activation of complement pathways after contusion-induced spinal cord injury. J Neurotrauma 2004; 21: 1831–1846.
O’Barr S, Yu JX, Cooper NR . Neuronal expression of the C5a receptor. Mol Immunol 1998; 35: 26.
Kim GH, Mocco J, Hahn DK, Kellner CP, Komotar RJ, Ducruet AF et al. Protective effect of C5a receptor inhibition after murine reperfused stroke. Neurosurgery 2008; 63: 122.
Woodruff TM, Costantini KJ, Crane JW, Atkin JD, Monk PN, Taylor SM et al. The complement factor C5a contributes to pathology in a rat model of amyotrophic lateral sclerosis. J Immunol 2008; 181: 8727–8734.
Busche MN, Stahl GL . Role of the complement components C5 and C3a in a mouse model of myocardial ischemia and reperfusion injury. GMS German Medical Science 2010; 8: 695–698.
Zivin JA, DeGirolami U . Spinal cord infarction: a highly reproducible stroke model. Stroke 1980; 11: 200–202.
Lemmon VP, Ferguson AR, Popovich PG, Xu XM, Snow DM, Igarashi M et al. Minimum information about a spinal cord injury experiment: a proposed reporting standard for spinal cord injury experiments. J Neurotrauma 2014; 31: 1354–1361.
Basso DM, Beattie MS, Bresnahan JC . A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 1995; 12: 1–21.
Kreutzberg GW . Microglia: a sensor for pathological events in the CNS. Trends Neurosci 1996; 19: 312–318.
Gasque P, Singhrao SK, Neal JW, Götze O, Morgan BP . Expression of the receptor for complement C5a C5aR) is up-regulated on reactive astrocytes, microglia, and endothelial cells in the inflamed human central nervous system. Am J Pathol 1997; 150: 31.
Van Beek J, Bernaudin M, Petit E, Gasque P, Nouvelot A, MacKenzie ET et al. Expression of receptors for complement anaphylatoxins C3a and C5a following permanent focal cerebral ischemia in the mouse. Exp Neurol 2000; 161: 373–382.
Ager RR, Fonseca MI, Chu SH, Sanderson SD, Taylor SM, Woodruff TM et al. Microglial C5aR (C5aR) expression correlates with amyloid-beta deposition in murine models of Alzheimer's disease. J Neurochem 2010; 113: 389–401.
Arumugam TV, Woodruff TM, Stocks SZ, Proctor LM, Pollitt S, Shiels IA et al. Protective effect of a human C5a receptor antagonist against hepatic ischaemia-reperfusion injury in rats. J Hepatol 2004; 40: 934–941.
Arumugam TV, Shiels IA, Strachan AJ, Abbenante G, Fairlie DP, Taylor SM . A small molecule C5a receptor antagonist protects kidneys from ischemia/reperfusion injury in rats. Kidney Int 2003; 63: 134–142.
Woodruff TM, Arumugam TV, Shiels IA, Reid RC, Fairlie DP, Taylor SM . Protective effects of a potent C5a receptor antagonist on experimental acute limb ischemia-reperfusion in rats. J Surg Res 2004; 116: 81–90.
Arumugam TV, Shiels IA, Woodruff TM, Reid RC, Fairlie DP, Taylor SM . Protective effect of a new c5a receptor antagonist against ischemia-reperfusion injury in the rat small intestine. J Surg Res 2002; 103: 260–267.
Okajima K . Prevention of endothelial cell injury by activated protein C: the molecular mechanism(s) and therapeutic implications. Curr Vasc Pharmacol 2004; 2: 125–133.
Oruckaptan HH, Ozisik P, Atilla P, Tuncel M, Kilinc K, Geyik PO et al. Systemic administration of interleukin-10 attenuates early ischemic response following spinal cord ischemia reperfusion injury in rats. J Surg Res 2009; 155: 345–356.
Tikka T, Fiebich BL, Goldsteins G, Keinanen R, Koistinaho J . Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. J Neurosci 2001; 21: 2580–2588.
Zhu P, Li JX, Fujino M, Zhuang J, Li XK . Development and treatments of inflammatory cells and cytokines in spinal cord ischemia-reperfusion injury. Mediators Inflamm 2013; 7: 701–709.
Acknowledgements
The Natural Science Foundation of China (81271339) contributed to this research.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Dong, Q., Sun, L., Peng, L. et al. PMX53 protects spinal cord from ischemia-reperfusion injury in rats in the short term. Spinal Cord 54, 254–258 (2016). https://doi.org/10.1038/sc.2015.146
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/sc.2015.146
This article is cited by
-
Mass cytometric analysis of the immune cell landscape after traumatic brain injury elucidates the role of complement and complement receptors in neurologic outcomes
Acta Neuropathologica Communications (2023)