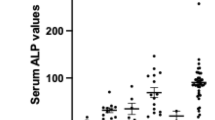
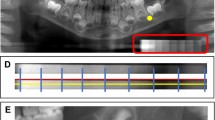
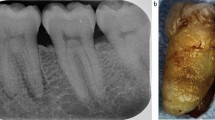
Key Points
-
Describes the clinical and dental features of Hypophosphatasia.
-
Provides a differential diagnosis for early loss of primary teeth.
-
Encourages GDP's to consider Hypophosphatasia when encountering patients with early loss of primary teeth.
-
Discusses new treatments available which may impact on the prognosis and treatment of patients with Hypophosphatasia
Abstract
Hypophosphatasia (HPP) is an inherited metabolic disorder that results in poorly mineralised bones and teeth. Clinical symptoms vary widely from mild dental anomalies to fatal fetal defects. The most common dental symptoms include exfoliation of the primary incisors before the age of three with little or no root resorption, large pulp chambers, alveolar bone loss and thin dentinal walls. There is generally minimal periodontal inflammation associated with the bony destruction and tooth loss. The general dental practitioner is usually the first clinician to spot signs of the milder forms of HPP. Patients diagnosed with dental symptoms in childhood can go on to develop significant morbidity in middle age with chronic bone pain and stress fractures of the long bones. The primary dental care clinician is the key to early diagnosis of such cases, whether they present in childhood or adulthood. Emerging enzyme replacement therapy has considerably changed the landscape of the disease, resulting in astonishing improvements in bone mineralisation and a significant reduction in mortality and morbidity. It is increasingly likely that primary and secondary care clinicians will treat patients with the severe forms of HPP, who would previously not have survived infancy.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Bishop N . Clinical management of hypophosphatasia. Clin Cases Miner Bone Metab 2015; 12: 170–173.
Whyte M P, Greenberg C R, Salman N J et al. Enzyme-replacement therapy in life-threatening hypophosphatasia. N Engl J Med 2012; 366: 904–913.
Hofmann C, Girschick H J, Mentrup B et al. Clinical Aspects of Hypophosphatasia: An Update. Clin Rev Bone Min Metabol 2013; 11: 60–70.
Bloch-Zupan A . Hypophosphatasia: diagnosis and clinical signs – a dental surgeon perspective. Int J Paediatr Dent 2016; 26: 426–438.
Whyte M, Leung E, Wilcox W et al. Hypophosphatasia: a retrospective natural history study of the severe perinatal and infantile forms. Bone Abstracts 2014; 3: 364.
Leung E C, Mhanni A A, Reed M, Whyte M P, Landy H, Greenberg C R . Outcome of perinatal hypophosphatasia in manitoba mennonites: a retrospective cohort analysis. JIMD Reports 2013; 11: 73–78.
Lynch C D, Ziada H M, Buckley LA, O'Sullivan V R, Aherne T, Aherne S . Prosthodontic rehabilitation of hypophosphatasia using dental implants: a review of the literature and two case reports. J Oral Rehabil 2009; 36: 462–468.
Whyte M P, Zhang F, Wenkert D et al. Hypophosphatasia: validation and expansion of the clinical nosology for children from 25 years experience with 173 paediatric patients. Bone 2015; 75: 229–239.
Rockman-Greenberg C . Hypophosphatasia. Pediatr Endocrinol Rev 2013; 10 Suppl 2: 380–388.
Reibel A, Maniere M C, Clauss F et al. Orodental phenotype and genotype findings in all subtypes of hypophosphatasia. Orphanet J Rare Dis 2009; 4: 6.
Mori M, DeArmey S L, Weber T J, Kishnani P S . Case series: Odontohypophosphatasia or missed diagnosis of childhood/adult-onset hypophosphatasia? – Call for a long-term follow-up of premature loss of primary teeth. Bone Reports 2016; 5: 228–232.
Chapple I L . Hypophosphatasia: dental aspects and mode of inheritance. J Clin Periodontol 1993; 20: 615–622.
Beck C, Morbach H, Richl P, Stenzel M, Girschick H J . How can calcium pyrophosphate crystals induce inflammation in hypophosphatasia or chronic inflammatory joint diseases? Rheumatol Int 2009; 29: 229–238.
Beumer J, 3rd, Trowbridge H O, Silverman S, Jr ., Eisenberg E . Childhood hypophosphatasia and the premature loss of teeth. A clinical and laboratory study of seven cases. Oral Surg Oral Med Oral Pathol 1973; 35: 631–640.
Foster B L, Ramnitz M S, Gafni R I et al. Rare bone diseases and their dental, oral, and craniofacial manifestations. J Dent Res 2014; 93 (7 Suppl): 7S–19S.
van den Bos T, Handoko G, Niehof A et al. Cementum and Dentin in Hypophosphatasia. J Dent Res 2005; 84: 1021–1025.
Mornet E, Yvard A, Taillandier A, Fauvert D, Simon-Bouy B . A molecular-based estimation of the prevalence of hypophosphatasia in the European population. Ann Hum Genet 2011; 75: 439–445.
Taketani T, Onigata K, Kobayashi H, Mushimoto Y, Fukuda S, Yamaguchi S . Clinical and genetic aspects of hypophosphatasia in Japanese patients. Arch Dis Child 2014; 99: 211–215.
Fraser D . Hypophosphatasia. Am J Med 1957; 22: 730–746.
Mornet E . Hypophosphatasia. Orphanet J Rare Dis 2007; 2: 40.
Whyte M P . Hypophosphatasia and the role of alkaline phosphatase in skeletal mineralization. Endocr Rev 1994; 15: 439–461.
Hofmann C, Girschick H, Mornet E, Schneider D, Jakob F, Mentrup B . Unexpected high intrafamilial phenotypic variability observed in hypophosphatasia. Eur J Hum Genet 2014; 22: 1160–1164.
Liu H, Li J, Lei H, Zhu T, Gan Y, Ge L . Genetic aetiology and dental pulp cell deficiency of hypophosphatasia. J Dent Res 2010; 89: 1373–1377.
Foster B L, Nagatomo K J, Nociti F H, Jr . et al. Central role of pyrophosphate in acellular cementum formation. PloS One 2012; 7: e38393.
Cole D E . Hypophosphatasia update: recent advances in diagnosis and treatment. Clin Genet 2008; 73: 232–235.
Demirbilek H, Alanay Y, Alikasifoglu A et al. Hypophosphatasia presenting with pyridoxine-responsive seizures, hypercalcaemia, and pseudotumour cerebri: case report. J Clin Res Pediatr Endocrinol 2012; 4: 34–38.
Fonta C, Barone P, Rodriguez Martinez L, Negyessy L . Rediscovering TNAP in the Brain: A Major Role in Regulating the Function and Development of the Cerebral Cortex. Subcell Biochem 2015; 76: 85–106.
Whyte M P, Wenkert D, McAlister W H et al. Chronic recurrent multifocal osteomyelitis mimicked in childhood hypophosphatasia. J Bone Miner Res 2009; 24: 1493–1505.
Hollis A, Arundel P, High A, Balmer R . Current concepts in hypophosphatasia: case report and literature review. Int J Paediatr Dent 2013; 23: 153–159.
Whyte M P, Rockman-Greenberg C, Ozono K et al. Asfotase Alfa Treatment Improves Survival for Perinatal and Infantile Hypophosphatasia. J Clin Endocrinol Metab 2016; 101: 334–342.
Gasque K C, Foster B L, Kuss P et al. Improvement of the skeletal and dental hypophosphatasia phenotype in Alpl/mice by administration of soluble (non-targeted) chimeric alkaline phosphatase. Bone 2015; 72: 137–147.
NICE. New Stensiq deal paves way for NICE approval of life saving drug. 2017. Available at https://www.nice.org.uk/news/article/stensiq-new-deal-paves-way-for-nice-approval-of-life-saving-drug (accessed May 2018).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Feeney, C., Stanford, N., Lee, S. et al. Hypophosphatasia and the importance of the general dental practitioner – a case series and discussion of upcoming treatments. Br Dent J 224, 937–943 (2018). https://doi.org/10.1038/sj.bdj.2018.441
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/sj.bdj.2018.441
This article is cited by
-
The diagnosis of hypophosphatasia in children as a multidisciplinary effort: an expert opinion
Journal of Endocrinological Investigation (2023)
-
The impact of enzyme replacement therapy on the oral health manifestations of hypophosphatasia among children: a scoping review
European Archives of Paediatric Dentistry (2023)
-
Evaluation of alveolar bone hypomineralization in pediatric hypophosphatasia using orthopantomography
Scientific Reports (2022)
-
Effects of Infantile Hypophosphatasia on Human Dental Tissue
Calcified Tissue International (2022)
-
Dental effects of enzyme replacement therapy in case of childhood-type hypophosphatasia
BMC Oral Health (2021)