Abstract
After orthotopic liver transplantation (OLT), patients with chronic hepatitis C virus (HCV) infection show nearly universal persistence of viremia and reinfection of the liver, but identifying the point at which the liver is reinfected morphologically can be difficult. One tool that may potentially be useful to detect reinfection is reverse transcriptase-polymerase chain reaction (RT-PCR), which has proven to be highly sensitive for detecting HCV RNA in formalin-fixed paraffin-embedded liver tissue. Our purpose was to gain insight into the time frame of HCV reinfection by assaying for HCV RNA in serial posttransplant liver biopsy specimens. Our study population consisted of 14 patients who underwent liver transplantation for hepatitis C and had confirmed HCV RNA in pretransplant serum, absence of HCV RNA in donor livers, and available consecutive posttransplant liver allograft specimens. We performed RT-PCR for HCV RNA in serial posttransplant liver biopsy specimens, beginning at 1 week until at least one biopsy from each tested positive. HCV RNA was detected in liver tissue by RT-PCR in 1-week post-OLT liver samples in 6 of 14 (42.8%) patients, the earliest being 5 days post-OLT. Eventually, each of the remaining eight samples became RT-PCR positive as well; the first detections occurred in these at 3 weeks (three cases), 4 weeks (three cases), 48 weeks (one case), and 144 weeks (one case). Histologic identification of hepatitis C recurrence was relatively insensitive in relation to these molecular data. These data suggest that (1) HCV RNA reinfection is nearly universal after liver transplantation in patients with chronic hepatitis C infection, (2) molecular reinfection by HCV occurs at a variable interval post-OLT, with the majority of allograft livers reinfected as early as 1 week, and (3) morphologic features of hepatitis C are usually appreciable at the time of “molecular” recurrence.
Similar content being viewed by others
INTRODUCTION
Hepatitis C virus (HCV) is one of the major causes of end stage liver disease worldwide and is a leading indication for orthotopic liver transplantation (OLT). HCV RNA has been successfully detected in serum (1, 2, 3, 4, 5) and fresh frozen (1, 6) and paraffin-embedded (6, 7, 8, 9, 10, 11, 12, 13, 14) tissue, using the sensitive technique of reverse transcriptase-polymerase chain reaction (RT-PCR).
Identification of virus in tissue is of particular relevance in the post-OLT setting. Although orthotopic liver transplantation has emerged as an effective treatment for end stage chronic hepatitis C, recurrent infection of the allograft occurs almost universally according to most authors (1, 2, 4, 5, 12, 15, 16, 17, 18, 19, 20, 21, 22, 23). The major supporting evidence has been the post-OLT persistence of HCV RNA in serum, detected using the PCR technique, in nearly all cases (3, 4, 18, 19, 24). Further evidence for the nearly universal recurrence of hepatitis C has been the detection of HCV RNA in liver tissue by RT-PCR in more than 90% of cases (12, 15, 23).
Despite the nearly universal recurrence of HCV viremia post-OLT, the frequency with which recurrent hepatitis is recognized histologically is only in approximately 50 to 70% of cases (5, 12, 21, 23), with differences possibly reflecting varying thresholds for diagnosis and follow-up intervals. Recurrent hepatitis C seems to begin as a lobular hepatitis that is typically recognized several months post-OLT (5, 12) and evolves to a chronic, portal-dominant hepatitis over the next several months. Greenson et al. noted acute, lobular hepatitis at an average of 135 days post-OLT (range, 39 to 279 days) and chronic hepatitis at an average of 356 days post OLT (range, 89 to 1365 days) (12). There is evidence that the acute, lobular hepatitis may occur subtly as early as 1 week post-OLT (KP Batts et al,. unpublished data). Others have reported histological evidence of onset as early as 4 weeks (4, 15) to as late as 3 years post-OLT (22).
To gain insight into the time frame for the “molecular” reinfection and histologic recurrence of hepatitis C, we retrospectively reviewed serial post-OLT liver samples from hepatitis C patients, using both RT-PCR and light microscopy.
MATERIALS AND METHODS
Specimens and Study Design
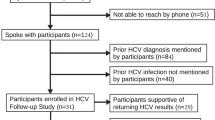
We retrospectively selected 14 patients who had (1) RT-PCR proven HCV RNA in pre-OLT serum and explanted liver tissue, (2) pretransplant donor biopsy specimens in which RT-PCR failed to detect HCV RNA, and (3) multiple post-OLT liver samples available for analysis (Fig. 1). A post-OLT liver biopsy sample from each patient was analyzed by RT-PCR for the presence of HCV RNA until at least one sample tested positive. If subsequent samples were available, one or more of those samples were also analyzed to determine the reproducibility of positivity (see below and Fig. 1). The histology slides from each analyzed episode were also reviewed by two experienced hepatic pathologists.
HCV RNA Detection
Fixation and storage of specimens
All biopsy specimens had been immersed in 10% neutral buffered formalin (CMS Fisher, Wood Dell, IL) overnight (6 to 24 h), and then processed through graded alcohols and paraffin infusion (Processor MVP, RMC Inc., Tucson, AZ). Infusion paraffin (CMS Fisher) was used for tissue infiltration, while embedding-type paraffin (Surgipath, Richmond, IL) was used for embedding. The paraffin embedded blocks were then stored at room temperature, whereas serum samples were stored at −70° C until the time of this study.
RNA extraction
For the tissue specimens, we used two unstained 5-μm sections of formalin-fixed paraffin-embedded liver tissue per sample, whereas 100-μl aliquots of serum were used for the serum specimens per sample. The tissue samples were transferred from glass slides to 1.5-mL microcentrifuge tubes containing 1 mL of RNAzol B (Leedo Laboratories, Houston, TX). The tubes were incubated at 70° C for 15 min to melt the paraffin and were vortexed briefly before the addition of 100 μL of RNAse-free water (USB, Cleveland, OH). Tissue samples were digested by incubation at room temperature for 1.5 h with vortexing every 30 min. The serum samples were transferred directly to 1.5-mL microcentrifuge tubes containing 1 mL of RNAzol B (Leedo Laboratories). From this point on, the preparation for both types of specimens remained the same. The extraction was performed using the same procedure described in our previous articles (13, 14).
RT-PCR
HCV RNA was amplified using the same combined RT-PCR method described previously (13, 14). The primers used target the high conserved 5′ untranslated region of the HCV.
Controls
For negative controls, we used formalin-fixed, paraffin-embedded liver tissue and serum specimens from two patients with a diagnosis of primary biliary cirrhosis and known to be serum and liver tissue HCV RNA negative by RT-PCR. Formalin-fixed, paraffin-embedded liver tissue and serum specimens from two patients known to be HCV positive in serum and liver tissue by RT-PCR were used as positive controls.
To further confirm a successful RNA extraction and the presence of intact RNA in each specimen, we performed RT-PCR for albumin mRNA in all cases. The positive albumin mRNA control consisted of liver tissue from a patient with primary biliary cirrhosis, whereas the negative albumin mRNA control consisted of human brain tissue. These controls were assayed parallel with study specimens.
Histologic Examination
Existing hematoxylin and eosin-stained slides from all post-OLT serial biopsy specimens that were tested by RT-PCR were reviewed independently by two experienced pathologists (KPB, LJB). Each was unaware of the time post-OLT, patient identity, and the molecular (HCV RNA) status of the specimen. In addition, two post-OLT cases known to be HCV RNA negative, with serial posttransplant biopsy specimens obtained between posttransplant day 7 and 4 months, were reviewed as negative controls. For each case, the degree of portal inflammation, piecemeal necrosis (interface hepatitis), lobular necrosis, and confluent necrosis was graded using the modified hepatic activity index of Ishak et al. (25). Macrovesicular steatosis was also graded on a 0 to 4 scale, corresponding to none (absent), minimal (<5% acinar tissue), mild (5 to 25%), moderate (25 to 50%), or marked (>50%) lobular involvement.
A subjective determination was made by a single reviewer (KPB) as to whether there appeared to be recurrent hepatitis C (yes, no, indeterminate). If yes or indeterminate, the dominant morphologic pattern was recorded as either lobular, periportal, or portal hepatitis, as described previously (26), corresponding to an appearance suggesting an “acute,” “chronic active,” or “chronic persistent” hepatitis pattern, respectively (27).
RESULTS
Molecular Study
HCV RNA in liver tissue
HCV RNA was detected by RT-PCR in specimens obtained approximately 1 week (5 to 9 days) post-OLT in 6 of 14 (42.8%) cases. Another four cases became positive at approximately 3 weeks post-OLT, and the remaining cases were noted to be positive at 4 weeks, 1 year, and 3 years post-OLT, as shown in Figure 1. Albumin mRNA was detected in all samples, confirming a successful extraction and the presence of intact RNA in the formalin-fixed, paraffin-embedded liver tissues. Positive and negative controls reacted appropriately. To further confirm the presence of the HCV RNA in the cases, we performed RT-PCR on subsequent biopsy specimens after the first positive result in nearly all of the cases. These samples were all positive for HCV RNA.
HCV RNA in serum
We detected HCV RNA in all of the post-OLT serum samples obtained the same day as the biopsy specimens. These samples were uniformly positive, independent of the biopsy HCV status. Positive and negative controls reacted appropriately.
Histologic Study
The frequencies at which piecemeal necrosis (interface hepatitis), lobular hepatitis, portal inflammation, and steatosis were noted in HCV RNA positive and negative biopsy specimens at the various post-OLT time intervals are shown in Figures 2, 3, 4, and 5. Piecemeal necrosis was thought to be present in only 4 of 14 1- or 3-week post-OLT samples that were HCV positive and was present in 3 of 14 1- or 2-week HCV-negative cases (Fig. 2), suggesting that piecemeal necrosis is not a reliable correlate of HCV RNA status in the immediate post-OLT period. In specimens taken 3 weeks post-OLT and beyond, none of the HCV negative specimens demonstrated piecemeal necrosis; however, even in the HCV-positive samples, piecemeal necrosis was present in only a minority (8 of 33; 24.2%). Interestingly, none of the three HCV-positive samples obtained 1 year and beyond showed piecemeal necrosis.
Lobular hepatitis was present in the majority of 1-week post-OLT samples regardless of HCV RNA status (Fig. 3), suggesting that, although this could be a reflection of early recurrent hepatitis C, it may in many cases reflect perioperative injury, perhaps related to preservation injury. In the 3-week to 6-month post-OLT period, 15 of 24 (63%) HCV-positive samples demonstrated lobular hepatitis versus 1 of 6 (17%) HCV-negative samples. In this time frame, the presence of lobular hepatitis (in patients undergoing transplantation for hepatitis C) had positive and negative predictive values for HCV positivity of 93.7% and 35.7%. None of the four 1-year or later post-OLT samples in our series showed a lobular hepatitis component, regardless of HCV RNA status.
Portal inflammation was present at 1-week post-OLT in approximately 50% of cases, regardless of HCV RNA status (Fig. 4). In the 1- to 6-month post-OLT time frame, 7 of 13 (54%) of HCV-positive specimens showed portal inflammation, and neither of the 2 HCV negative specimens had significant portal inflammation. As with both piecemeal necrosis and lobular hepatitis, there was no portal inflammation in the four 1-year or later post-OLT samples, regardless of HCV status.
Steatosis, generally mild, was present in most biopsy samples obtained 3 weeks or later post-OLT in both groups, suggesting that it is not a useful predictor of tissue HCV RNA status post-OLT (Fig. 5). Among the HCV RNA-positive cases, steatosis was only observed in 1 of 6 samples in the first week post-OLT, suggesting that if steatosis is a feature of hepatitis C post-OLT, it probably takes time to manifest.
We also specifically analyzed the morphologic appearance of the first HCV RNA-positive liver specimens for each case (Table 1). At least one of the reviewers noted lobular hepatitis in 9 of 14 cases; the cases lacking lobular hepatitis occurred at 8 days, 3 and 4 weeks, and 1 and 3 years. Piecemeal necrosis and significant portal inflammation were uncommon, supporting that early recurrent hepatitis C, when evident at all, will be in the form of an acute, lobular hepatitis rather than a “chronic, active” hepatitis. The presence of steatosis was uncommon at the time of the first positive HCV RNA sample, particularly in the first several months post-OLT. Subjectively, definite morphologic features of recurrent hepatitis C were difficult to appreciate, with only 2 of 14 (14%) cases considered likely to show recurrence, 5 of 14 (36%) showing equivocal recurrence, and 7 of 14 (50%) showing no likely recurrence.
Neither of the 2 negative control cases were thought to have morphologic features of recurrent hepatitis C on blind review. Neither case showed lobular necrosis or portal inflammation at 3 weeks or 4 months, and both showed trivial periportal inflammation (piecemeal necrosis) at 4 months, which was not considered significant.
DISCUSSION
RT-PCR has proved to be a sensitive and specific method of detecting HCV RNA (28). This technique readily detects HCV RNA in serum, fresh frozen liver tissue, and formalin-fixed paraffin-embedded liver tissue (6, 11, 12, 15, 28, 29, 30). Interestingly, HCV-RNA has also been detected in nonhepatic reservoirs, such as those of the lung, testis, cerebellum, adrenal, and kidney (31, 32, 33). A possible explanation for these observations could be the detection of HCV RNA in serum or peripheral blood mononuclear cells, which accompany the tissue specimen (34, 35). Another observation that raises the possibility of tissue RT-PCR detection of circulating HCV RNA has been the discovery of HCV RNA in liver biopsy tissue as early as 7 to 12 days post-OLT (5, 22). In a preliminary study, however, we failed to detect HCV RNA by RT-PCR in liver biopsies taken from HCV-positive individuals immediately posttransplant (14). Our inability to detect HCV RNA by RT-PCR in these specimens indicates that, at least in our system, “background” peripheral blood in liver biopsy specimens does not yield false positivity that theoretically could have been caused by “passenger” HCV RNA in serum or peripheral blood mononuclear cells that accompany the tissue. We have previously shown that this technique in similarly fixed (overnight formalin) tissue from the explanted liver specimens of these patients showed 100% sensitivity and yielded no false positives (13, 14). The above results are important in validating the use of RT-PCR to detect HCV RNA in tissue, particularly given the technical difficulties to date using immunoperoxidase (29, 36, 37) and in situ hybridization techniques (37, 38, 39).
The exact replication mechanism of the HCV is not completely understood. The presence of the negative RNA strand is regarded as proof of viral replication and has been demonstrated in liver tissue, confirming the hepatotropism of the virus (35). Because we did not seek the negative RNA strand in this study, we cannot comment on the presence of viral replication. Nevertheless, given the specimens’ persistently testing positive after the initial positive result and the absence of detectable HCV RNA in “Day 0” specimens (14), we believe that our detection of HCV RNA in posttransplant liver biopsy specimens as early as 5 days most likely reflects true hepatic infection.
Persistence of HCV RNA in serum post-OLT is well documented (18, 19, 40). Whereas in the nontransplant setting, the usual minimum clinical incubation period for hepatitis C is usually 6 to 7 weeks (23). As mentioned above, prior studies have identified HCV RNA in liver tissue as early as 1 week post-OLT (5, 22), suggesting, not unexpectedly, that the infection of hepatocytes by HCV occurs well before clinical symptoms are manifest. Our identification of HCV RNA in many 1-week post-OLT biopsies confirms these findings. Our findings that not all biopsy samples appear to be infected by 1 week and that some do not contain identifiable HCV RNA until 3 years post-OLT suggest that infection occurs at a variable rate post-OLT. Additional studies will be necessary to determine if the time to “molecular” recurrence is related to pretransplant HCV genotype, HCV RNA levels, host factors, or some combination of these.
The histologic portion of this study was designed to determine whether, in patients in whom recurrence of hepatitis C is anticipated, HCV RNA status of the biopsy sample resulted in recognizable histologic differences when comparing samples taken at a similar point in time post-OLT. We did not identify reliable histologic predictors of HCV RNA status among these patients. One week samples demonstrated similar degrees of piecemeal necrosis, lobular hepatitis, portal inflammation, and steatosis, and in individual cases, recurrent hepatitis C was difficult to identify with certainty (Fig. 6). This may reflect superimposed preservation and/or rejection changes that may obscure the histology changes of recurrent hepatitis C. In samples taken more than 1 month post-OLT, lobular hepatitis was observed in approximately two-thirds of HCV RNA-positive samples but was not seen in HCV negative samples, suggesting that lobular hepatitis occurring after the immediate posttransplantation period may be a helpful but insensitive indicator of tissue HCV RNA positivity (Fig. 7). The very few 1 year and beyond samples examined here did not provide convincing evidence of recurrent hepatitis C histologically. This is similar to prior studies that demonstrated morphologic evidence of recurrent hepatitis C in only 50 to 70% of cases (5, 12, 21, 23). Although this study was not designed to trace the morphologic evolution of recurrent hepatitis C, there seems to be a tendency for portal inflammation to manifest in the 1–6 month period among HCV-positive samples, suggesting evolution to a “chronic, active hepatitis” pattern as described by prior authors (12).
Histopathology of recurrent hepatitis C at 1 week posttransplantation. The liver is histologically unremarkable despite positive hepatitis C virus RNA testing by polymerase chain reaction analysis of biopsy tissue. Lack of histologic evidence of recurrent hepatitis is common at 1 week after orthotopic liver transplantation, presumably reflecting very early, histologically inapparent infection (hematoxylin and eosin).
Histopathology of recurrent hepatitis C at 6 weeks posttransplantation. There is mild lobular hepatitis with acidophil bodies (arrow) and mild steatosis, which is histologically characteristic of early recurrent hepatitis C. This patient’s Day 7 biopsy sample, as well as this sample, were hepatitis C virus RNA positive by tissue polymerase chain reaction analysis (hematoxylin and eosin).
In summary, we have shown that reinfection of hepatic allografts occurs commonly within 1 week posttransplantation and usually 1 month, with occasional late recurrence at 1 or more years posttransplantation. Additional study with greater numbers of cases will be necessary to identify risk factor for early molecular recurrence of hepatitis C and the clinical significance of early versus late molecular recurrence. At this point, it is not clear how HCV RNA status of liver biopsy specimens should impact medical management. We also found that definite morphologic evidence of recurrence of hepatitis C is generally not present at the time of molecular recurrence. Presence of lobular hepatitis and piecemeal necrosis, after the first few weeks posttransplantation, are somewhat helpful yet insensitive indicators of tissue HCV RNA positivity.
References
Gretch DR, Bacchi CE, Corey L, dela Rosa C, Lesniewski RR, Kowdley K, et al. Persistent hepatitis C virus infection after liver transplantation: clinical and virological features. Hepatology 1995; 22: 1–9.
Belli LS, Silini E, Alberti A, Bellati G, Vai C, Minola E, et al. Hepatitis C virus genotypes, hepatitis, and hepatitis C virus recurrence after liver transplantation. Liver Transpl Surg 1996; 2: 200–205.
Donegan E, Wright TL, Roberts J, Ascher NL, Lake JR, Neuwald P, et al. Detection of hepatitis C after liver transplantation. Four serologic tests compared. Am J Clin Pathol 1995; 104: 673–679.
Feray C, Gigou M, Samuel D, Paradis V, Wilber J, David MF, et al. The course of hepatitis C virus infection after liver transplantation. Hepatology 1994; 20: 1137–1143.
Ferrell LD, Wright TL, Roberts J, Ascher N, Lake J . Hepatitis C viral infection in liver transplant recipients. Hepatology 1992; 16: 865–876.
Bresters D, Cuypers HT, Reesink HW, Chamuleau RA, Schipper ME, Boeser-Nunnink BD, et al. Detection of hepatitis C viral RNA sequences in fresh and paraffin-embedded liver biopsy specimens of non-A, non-B hepatitis patients. J Hepatol 1992; 15: 391–395.
Svoboda-Newman SM, Greenson JK, Singleton TP, Sun R, Frank TS . Detection of hepatitis C by RT-PCR in formalin-fixed paraffin-embedded tissue from liver transplant patients. Diagn Mol Pathol 1997; 6: 123–129.
Akyol G, Dash S, Shieh YS, Malter JS, Gerber MA . Detection of hepatitis C virus RNA sequences by polymerase chain reaction in fixed liver tissue. Mod Pathol 1992; 5: 501–504.
Park YN, Abe K, Li H, Hsuih T, Thung SN, Zhang DY . Detection of hepatitis C virus RNA using ligation-dependent polymerase chain reaction in formalin-fixed, paraffin-embedded liver tissues. Am J Pathol 1996; 149: 1485–1491.
Bresters D, Schipper ME, Reesink HW, Boeser-Nunnink BD, Cuypers HT . The duration of fixation influences the yield of HCV cDNA-PCR products from formalin-fixed, paraffin-embedded liver tissue. J Virol Methods 1994; 48: 267–272.
el-Batanony MH, Savage K, Jacobs R, el-Refaie AO, Squadrito GG, Brown D, et al. Hepatitis C virus-polymerase chain reaction of routinely processed liver biopsies. J Med Virol 1994; 43: 380–385.
Greenson JK, Svoboda-Newman SM, Merion RM, Frank TS . Histologic progression of recurrent hepatitis C in liver transplant allografts. Am J Surg Pathol 1996; 20: 731–738.
Guerrero RB, Batts KP, Brandhagen DJ, Germer JJ, Perez RG, Persing DH . Effects of formalin fixation and prolonged block storage on detection of hepatitis C virus RNA in liver tissue. Diagn Mol Pathol 1997; 6: 277–281.
Guerrero RB, Batts KP, Germer JJ, Perez RG, Wiesner RH, Persing DH . Reverse transcriptase-polymerase chain reaction fails to detect peripheral-blood hepatitis C RNA in formalin-fixed liver tissue. Liver Transpl Surg 1998; 4: 455–460.
Sallie R, Cohen AT, Tibbs CJ, Portmann BC, Rayner A, O'Grady JG, et al. Recurrence of hepatitis C following orthotopic liver transplantation: a polymerase chain reaction and histological study. J Hepatol 1994; 21: 536–542.
Knoop M, Bechstein WO, Blumhardt G, Langrehr JM, Berg T, Konig V, et al. Recurrent hepatitis C infection after orthotopic liver transplantation. Transplant Proc 1995; 27: 1208–1210.
Marzano A, Smedile A, Abate M, Ottobrelli A, Brunetto M, Negro F, et al. Hepatitis type C after orthotopic liver transplantation: reinfection and disease recurrence. J Hepatol 1994; 21: 961–965.
Villa E, Grottola A, Buttafoco P, Merighi A, Ferretti I, Trande P, et al. Long-term follow-up of hepatitis C virus (HCV) infection in liver transplant patients. Clin Transplant 1995; 9: 160–164.
Weinstein JS, Poterucha JJ, Zein N, Wiesner RH, Persing DH, Rakela J . Epidemiology and natural history of hepatitis C infections in liver transplant recipients. J Hepatol 1995; 22: 154–159.
Shah G, Demetris AJ, Gavaler JS, Lewis JH, Todo S, Starzl TE, et al. Incidence, prevalence, and clinical course of hepatitis C following liver transplantation. Gastroenterology 1992; 103: 323–329.
Shiffman ML, Contos MJ, Luketic VA, Sanyal AJ, Purdum PP III, Mills AS, et al. Biochemical and histologic evaluation of recurrent hepatitis C following orthotopic liver transplantation. Transplantation 1994; 57: 526–532.
Sheiner PA, Schwartz ME, Mor E, Schluger LK, Theise N, Kishikawa K, et al. Severe or multiple rejection episodes are associated with early recurrence of hepatitis C after orthotopic liver transplantation. Hepatology 1995; 21: 30–34.
Thung SN, Shim KS, Shieh YS, Schwartz M, Theise N, Borcich A, et al. Hepatitis C in liver allografts. Arch Pathol Lab Med 1993; 117: 145–149.
Fukumoto T, Berg T, Ku Y, Bechstein WO, Knoop M, Lemmens HP, et al. Viral dynamics of hepatitis C early after orthotopic liver transplantation: evidence for rapid turnover of serum virions. Hepatology 1996; 24: 1351–1354.
Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, et al. Histological grading and staging of chronic hepatitis. J Hepatol 1995; 22: 696–699.
Ludwig MD, Batts MD . Lobular hepatitis. In: Practical Liver Biopsy Interpretation (Diagnostic Algorithms). 2nd ed. Chicago: ASCP Press; 1998. p. 28–29.
Batts KP, Ludwig J . Chronic hepatitis. An update on terminology and reporting. Am J Surg Pathol 1995; 19: 1409–1417.
Cuypers HT, Bresters D, Winkel IN, Reesink HW, Weiner AJ, Houghton M, et al. Storage conditions of blood samples and primer selection affect the yield of cDNA polymerase chain reaction products of hepatitis C virus. J Clin Microbiol 1992; 30: 3220–3224.
Sansonno D, Iacobelli AR, Cornacchiulo V, Distasi M, Dammacco F . Immunohistochemical detection of hepatitis C virus-related proteins in liver tissue. Clin Exp Rheumatol 1995; 13 (Suppl 13): S29–S32.
Tanaka Y, Enomoto N, Kojima S, Tang L, Goto M, Marumo F, et al. Detection of hepatitis C virus RNA in the liver by in situ hybridization. Liver 1993; 13: 203–208.
Bolay H, Soylemezoglu F, Nurlu G, Tuncer S, Varli K . PCR detected hepatitis C virus genome in the brain of a case with progressive encephalomyelitis with rigidity. Clin Neurol Neurosurg 1996; 98: 305–308.
Tang D, Jia T, Brandwein M, Thung SN, Zhang DY . Detection of HCV RNA in various organs using novel ligation-dependent polymerase chain reaction (LD-PCR). Mod Pathol 1996; 9: 131A.
Takehara T, Hayashi N, Mita E, Hagiwara H, Ueda K, Katayama K, et al. Detection of the minus strand of hepatitis C virus RNA by reverse transcription and polymerase chain reaction: implications for hepatitis C virus replication in infected tissue. Hepatology 1992; 15: 387–390.
Chang TT, Young KC, Yang YJ, Lei HY, Wu HL . Hepatitis C virus RNA in peripheral blood mononuclear cells: comparing acute and chronic hepatitis C virus infection. Hepatology 1996; 23: 977–981.
Fong TL, Shindo M, Feinstone SM, Hoofnagle JH, Di Bisceglie AM . Detection of replicative intermediates of hepatitis C viral RNA in liver and serum of patients with chronic hepatitis C. J Clin Invest 1991; 88: 1058–1060.
Kojima S, Tanaka Y, Enomoto N, Marumo F, Sato C . Distribution of hepatitis C virus RNA in the liver and its relation to histopathological changes. Liver 1996; 16: 55–60.
Haruna Y, Hayashi N, Hiramatsu N, Takehara T, Hagiwara H, Sasaki Y, et al. Detection of hepatitis C virus RNA in liver tissues by an in situ hybridization technique. J Hepatol 1993; 18: 96–100.
Cho SW, Hwang SG, Han DC, Jin SY, Lee MS, Shim CS, et al. In situ detection of hepatitis C virus RNA in liver tissue using a digoxigenin-labeled probe created during a polymerase chain reaction. J Med Virol 1996; 48: 227–233.
Lidonnici K, Lane B, Nuovo GJ . Comparison of serologic analysis and in situ localization of PCR-amplified cDNA for the diagnosis of hepatitis C infection. Diagn Mol Pathol 1995; 4: 98–107.
Terrault NA, Wright TL, Pereira BJ . Hepatitis C infection in the transplant recipient. Infect Dis Clin North Am 1995; 9: 943–964.
Acknowledgements
This material has been presented in poster format at the 1999 United States and Canadian Academy of Pathology meeting in San Francisco, California on March, 27, 1999. We thank Crystal Holtz for assistance in preparing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guerrero, R., Batts, K., Burgart, L. et al. Early Detection of Hepatitis C Allograft Reinfection after Orthotopic Liver Transplantation: A Molecular and Histologic Study. Mod Pathol 13, 229–237 (2000). https://doi.org/10.1038/modpathol.3880043
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/modpathol.3880043
Keywords
This article is cited by
-
HCV in liver transplantation
Seminars in Immunopathology (2013)
-
What is expected from the pathologist in the diagnosis of viral hepatitis?
Virchows Archiv (2011)
-
The clinical consequences of utilizing donation after cardiac death liver grafts into hepatitis C recipients
Hepatology International (2011)
-
Approach to recurrent hepatitis C following liver transplantation
Current Gastroenterology Reports (2007)