Abstract
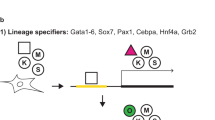
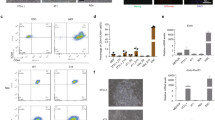
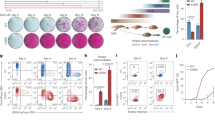
Ectopic expression of defined sets of transcription factors in somatic cells enables them to adopt the qualities of pluripotency. Mouse embryonic fibroblasts (MEFs) are the classic target cell used to elucidate the core principles of nuclear reprogramming. However, their phenotypic and functional heterogeneity represents a major hurdle for mechanistic studies aimed at defining the molecular nature of cellular plasticity. We show that reducing the complexity of MEFs by flow cytometry allows the isolation of discrete cell subpopulations that can be efficiently reprogrammed to pluripotency with fewer genes. Using these FACS-sorted cells, we performed a systematic side-by-side analysis of the reprogramming efficiency with different two- and three-factor combinations of Oct4, Sox2 and Klf4. We show that introduction of exogenous Oct4 with either Sox2 or Klf4 does not directly convert MEFs to a pluripotent state. Instead, each combination of factors disrupts the normal cellular homeostasis and establishes transient states characterized by the concurrent expression of mixed lineage markers. These cells convert into induced pluripotent stem cells in a stochastic fashion. Our data suggest that there is a partial functional redundancy between Sox2 and Klf4 in the disruption of cellular homeostasis and activation of regulatory networks that define pluripotency.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- FACS:
-
fluorescence-activated cell sorting
- GFP:
-
green fluorescent protein
- iPS cells:
-
induced pluripotent stem cells
- KSR:
-
knockout serum replacement
- MEFs:
-
mouse embryonic fibroblasts
- OKSM:
-
Oct4, Klf4, Sox2 and c-Myc
References
Hanna JH, Saha K, Jaenisch R . Pluripotency and cellular reprogramming: facts, hypotheses, unresolved issues. Cell 2010; 143: 508–525.
Stadtfeld M, Hochedlinger K . Induced pluripotency: history, mechanisms, and applications. Genes Dev 2010; 24: 2239–2263.
Takahashi K, Yamanaka S . Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006; 126: 663–676.
Maherali N, Sridharan R, Xie W, Utikal J, Eminli S, Arnold K et al. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell 2007; 1: 55–70.
Wernig M, Meissner A, Foreman R, Brambrink T, Ku M, Hochedlinger K et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature 2007; 448: 318–324.
Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007; 318: 1917–1920.
Kim JB, Zaehres H, Wu G, Gentile L, Ko K, Sebastiano V et al. Pluripotent stem cells induced from adult neural stem cells by reprogramming with two factors. Nature 2008; 454: 646–650.
Tsai SY, Clavel C, Kim S, Ang YS, Grisanti L, Lee DF et al. Oct4 and klf4 reprogram dermal papilla cells into induced pluripotent stem cells. Stem Cells 2010; 28: 221–228.
Kim JB, Greber B, Arauzo-Bravo MJ, Meyer J, Park KI, Zaehres H et al. Direct reprogramming of human neural stem cells by OCT4. Nature 2009; 461: 649–3.
Tsai SY, Bouwman BA, Ang YS, Kim SJ, Lee DF, Lemischka IR et al. Single transcription factor reprogramming of hair follicle dermal papilla cells to induced pluripotent stem cells. Stem Cells 2011; 29: 964–971.
Wernig M, Lengner CJ, Hanna J, Lodato MA, Steine E, Foreman R et al. A drug-inducible transgenic system for direct reprogramming of multiple somatic cell types. Nat Biotechnol 2008; 26: 916–924.
Markoulaki S, Hanna J, Beard C, Carey BW, Cheng AW, Lengner CJ et al. Transgenic mice with defined combinations of drug-inducible reprogramming factors. Nat Biotechnol 2009; 27: 169–171.
Eminli S, Utikal J, Arnold K, Jaenisch R, Hochedlinger K . Reprogramming of neural progenitor cells into induced pluripotent stem cells in the absence of exogenous Sox2 expression. Stem Cells 2008; 26: 2467–2474.
Stadtfeld M, Maherali N, Borkent M, Hochedlinger K . A reprogrammable mouse strain from gene-targeted embryonic stem cells. Nat Methods 2010; 7: 53–55.
Carey BW, Markoulaki S, Beard C, Hanna J, Jaenisch R . Single-gene transgenic mouse strains for reprogramming adult somatic cells. Nat Methods 2010; 7: 56–59.
Hanna J, Saha K, Pando B, van Zon J, Lengner CJ, Creyghton MP et al. Direct cell reprogramming is a stochastic process amenable to acceleration. Nature 2009; 462: 595–601.
Hanna J, Carey BW, Jaenisch R . Reprogramming of somatic cell identity. Cold Spring Harb Symp Quant Biol 2008; 73: 147–155.
Nagy A, Nagy K . The mysteries of induced pluripotency: where will they lead? Nat Methods 2010; 7: 22–24.
Koumas L, Smith TJ, Feldon S, Blumberg N, Phipps RP . Thy-1 expression in human fibroblast subsets defines myofibroblastic or lipofibroblastic phenotypes. Am J Pathol 2003; 163: 1291–1300.
Phan SH . Biology of fibroblasts and myofibroblasts. Proc Am Thorac Soc 2008; 5: 334–337.
Mikkelsen TS, Hanna J, Zhang X, Ku M, Wernig M, Schorderet P et al. Dissecting direct reprogramming through integrative genomic analysis. Nature 2008; 454: 49–55.
Sridharan R, Tchieu J, Mason MJ, Yachechko R, Kuoy E, Horvath S et al. Role of the murine reprogramming factors in the induction of pluripotency. Cell 2009; 136: 364–377.
Li R, Liang J, Ni S, Zhou T, Qing X, Li H et al. A mesenchymal-to-epithelial transition initiates and is required for the nuclear reprogramming of mouse fibroblasts. Cell Stem Cell 2010; 7: 51–63.
Samavarchi-Tehrani P, Golipour A, David L, Sung HK, Beyer TA, Datti A et al. Functional genomics reveals a BMP-driven mesenchymal-to-epithelial transition in the initiation of somatic cell reprogramming. Cell Stem Cell 2010; 7: 64–77.
Petrenko O, Beavis A, Klaine M, Kittappa R, Godin I, Lemischka IR . The molecular characterization of the fetal stem cell marker AA4. Immunity 1999; 10: 691–700.
Sanders YY, Kumbla P, Hagood JS . Enhanced myofibroblastic differentiation and survival in Thy-1(-) lung fibroblasts. Am J Respir Cell Mol Biol 2007; 36: 226–235.
Holmes C, Stanford WL . Concise review: stem cell antigen-1: expression, function, and enigma. Stem Cells 2007; 25: 1339–1347.
Gu B, Zhang J, Wang W, Mo L, Zhou Y, Chen L et al. Global expression of cell surface proteins in embryonic stem cells. PLoS One 2010; 5: e15795.
Stadtfeld M, Maherali N, Breault DT, Hochedlinger K . Defining molecular cornerstones during fibroblast to iPS cell reprogramming in mouse. Cell Stem Cell 2008; 2: 230–240.
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 2005; 102: 15545–15550.
Wong DJ, Liu H, Ridky TW, Cassarino D, Segal E, Chang HY . Module map of stem cell genes guides creation of epithelial cancer stem cells. Cell Stem Cell 2008; 2: 333–344.
Kim J, Woo AJ, Chu J, Snow JW, Fujiwara Y, Kim CG et al. A Myc network accounts for similarities between embryonic stem and cancer cell transcription programs. Cell 2010; 143: 313–324.
Mignone JL, Kukekov V, Chiang AS, Steindler D, Enikolopov G . Neural stem and progenitor cells in nestin-GFP transgenic mice. J Comp Neurol 2004; 469: 311–324.
Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 2010; 466: 829–834.
Brambrink T, Foreman R, Welstead GG, Lengner CJ, Wernig M, Suh H et al. Sequential expression of pluripotency markers during direct reprogramming of mouse somatic cells. Cell Stem Cell 2008; 2: 151–159.
Smith ZD, Nachman I, Regev A, Meissner A . Dynamic single-cell imaging of direct reprogramming reveals an early specifying event. Nat Biotechnol 2010; 28: 521–526.
Nash KL, Lever AM . Green fluorescent protein: green cells do not always indicate gene expression. Gene Ther 2004; 11: 882–883.
MacArthur BD, Please CP, Oreffo RO . Stochasticity and the molecular mechanisms of induced pluripotency. PLoS One 2008; 3: e3086.
Loh KM, Lim B . A precarious balance: pluripotency factors as lineage specifiers. Cell Stem Cell 2011; 8: 363–369.
Li H, Collado M, Villasante A, Strati K, Ortega S, Canamero M et al. The Ink4/Arf locus is a barrier for iPS cell reprogramming. Nature 2009; 460: 1136–1139.
Acknowledgements
This work was supported by NYSTEM contract no. N08T-040 to UMM and OP. AN was supported by a fellowship from the Lymphoma Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by R De Maria
Supplementary Information accompanies the paper on Cell Death and Differentiation website
Supplementary information
Rights and permissions
About this article
Cite this article
Nemajerova, A., Kim, S., Petrenko, O. et al. Two-factor reprogramming of somatic cells to pluripotent stem cells reveals partial functional redundancy of Sox2 and Klf4. Cell Death Differ 19, 1268–1276 (2012). https://doi.org/10.1038/cdd.2012.45
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2012.45
Keywords
This article is cited by
-
Generation of a mouse SWATH-MS spectral library to quantify 10148 proteins involved in cell reprogramming
Scientific Data (2021)
-
A computational systems approach identifies synergistic specification genes that facilitate lineage conversion to prostate tissue
Nature Communications (2017)
-
ΔNp63 regulates select routes of reprogramming via multiple mechanisms
Cell Death & Differentiation (2013)
-
High-resolution analysis with novel cell-surface markers identifies routes to iPS cells
Nature (2013)
-
p73 is dispensable for commitment to neural stem cell fate, but is essential for neural stem cell maintenance and for blocking premature differentiation
Cell Death & Differentiation (2013)