Abstract
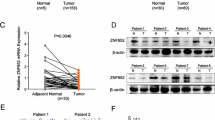
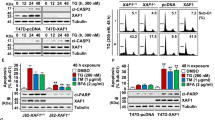
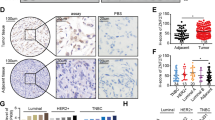
ZNF313 encoding a zinc-binding protein is located at chromosome 20q13.13, which exhibits a frequent genomic amplification in multiple human cancers. However, the biological function of ZNF313 remains largely undefined. Here we report that ZNF313 is an ubiquitin E3 ligase that has a critical role in the regulation of cell cycle progression, differentiation and senescence. In this study, ZNF313 is initially identified as a XIAP-associated factor 1 (XAF1)-interacting protein, which upregulates the stability and proapoptotic effect of XAF1. Intriguingly, we found that ZNF313 activates cell cycle progression and suppresses cellular senescence through the RING domain-mediated degradation of p21WAF1. ZNF313 ubiquitinates p21WAF1 and also destabilizes p27KIP1 and p57KIP2, three members of the CDK-interacting protein (CIP)/kinase inhibitor protein (KIP) family of cyclin-dependent kinase inhibitors, whereas it does not affect the stability of the inhibitor of CDK (INK4) family members, such as p16INK4A and p15INK4B. ZNF313 expression is tightly controlled during the cell cycle and its elevation at the late G1 phase is crucial for the G1-to-S phase transition. ZNF313 is induced by mitogenic growth factors and its blockade profoundly delays cell cycle progression and accelerates p21WAF1-mediated senescence. Both replicative and stress-induced senescence are accompanied with ZNF313 reduction. ZNF313 is downregulated during cellular differentiation process in vitro and in vivo, while it is commonly upregulated in many types of cancer cells. ZNF313 shows both the nuclear and cytoplasmic localization in epithelial cells of normal tissues, but exhibits an intense cytoplasmic distribution in carcinoma cells of tumor tissues. Collectively, ZNF313 is a novel E3 ligase for p21WAF1, whose alteration might be implicated in the pathogenesis of several human diseases, including cancers.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Abbreviations
- BMP:
-
bone morphogenetic protein
- CDK:
-
cyclin-dependent kinase
- CIP:
-
CDK-interacting protein
- IP:
-
immunoprecipitation
- INK4:
-
inhibitor of CDK
- KIP:
-
kinase inhibitor protein
- RT-PCR:
-
reverse transcription-polymerase chain reaction
- siRNA:
-
short-interfering RNA
- TGF:
-
transforming growth factor
- TRAIL:
-
TNF-related apoptosis-inducing ligand
- UIM:
-
ubiquitin-interacting motif
- XAF1:
-
XIAP-associated factor 1
References
Li N, Sun H, Wu Q, Tao D, Zhang S, Ma Y . Cloning and expression analysis of a novel mouse zinc finger protein gene Znf313 abundantly expressed in testis. J Biochem Mol Biol 2007; 40: 270–276.
Giannini AL, Gao Y, Bijlmakers MJ . T-cell regulator RNF125/TRAC-1 belongs to a novel family of ubiquitin ligases with zinc fingers and a ubiquitin-binding domain. Biochem J 2008; 410: 101–111.
Ma YX, Zhang SZ, Hou YP, Huang XL, Wu QQ, Sun Y . Identification of a novel human zinc finger protein gene ZNF313. Sheng wu hua xue yu sheng wu wu li xue bao (Shanghai) 2003; 35: 230–237.
Bijlmakers MJ, Kanneganti SK, Barker JN, Trembath RC, Capon F . Functional analysis of the RNF114 psoriasis susceptibility gene implicates innate immune responses to double-stranded RNA in disease pathogenesis. Hum Mol Genet 2011; 20: 3129–3137.
Fenner BJ, Scannell M, Prehn JHM . Identification of polyubiquitin binding proteins involved in NF-kappaB signaling using protein arrays. Biochim Biophys Acta 2009; 1794: 1010–1016.
Narayan G, Murty VV . Integrative genomic approaches in cervical cancer: implications for molecular pathogenesis. Future Oncol 2010; 6: 1643–1652.
Capon F, Bijlmakers M-J, Wolf N, Quaranta M, Huffmeier U, Allen M et al. Identification of ZNF313/RNF114 as a novel psoriasis susceptibility gene. Hum Mol Genet 2008; 17: 1938–1945.
Stuart PE, Nair RP, Ellinghaus E, Ding J, Tejasvi T, Gudjonsson JE et al. Genome-wide association analysis identifies three psoriasis susceptibility loci. Nat Genet 2010; 42: 1000–1004.
Lu Z, Hunter T . Degradation of activated protein kinases by ubiquitination. Annu Rev Biochem 2009; 78: 435–475.
Sherr CJ, Roberts JM . CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev 1999; 13: 1501–1512.
Bloom J, Pagano M . Deregulated degradation of the cdk inhibitor p27 and malignant transformation. Semin Cancer Biol 2003; 13: 41–47.
Malumbres M, Barbacid M . To cycle or not to cycle: a critical decision in cancer. Nat Rev Cancer 2001; 1: 222–231.
Liston P, Fong WG, Kelly NL, Toji S, Miyazaki T, Conte D et al. Identification of XAF1 as an antagonist of XIAP anti-caspase activity. Nat Cell Biol 2001; 3: 128–133.
Byun DS, Cho K, Ryu BK, Lee MG, Kang MJ, Kim HR et al. Hypermethylation of XIAP-associated factor 1, a putative tumor suppressor gene from the 17p13.2 locus, in human gastric adenocarcinomas. Cancer Res 2003; 63: 7068–7075.
Lee MG, Huh JS, Chung SK, Lee JH, Byun DS, Ryu BK et al. Promoter CpG hypermethylation and downregulation of XAF1 expression in human urogenital malignancies: implication for attenuated p53 response to apoptotic stresses. Oncogene 2006; 25: 5807–5822.
Chung SK, Lee MG, Ryu BK, Lee JH, Han J, Byun DS et al. Frequent alteration of XAF1 in human colorectal cancers: implication for tumor cell resistance to apoptotic stresses. Gastroenterology 2007; 132: 2459–2477.
Tu SP, Sun YW, Cui JT, Zou B, Lin MCM, Gu Q et al. Tumor suppressor XIAP-Associated factor 1 (XAF1) cooperates with tumor necrosis factor-related apoptosis-inducing ligand to suppress colon cancer growth and trigger tumor regression. Cancer 2010; 116: 1252–1263.
Micali OC, Cheung HH, Plenchette S, Hurley SL, Liston P, LaCasse EC et al. Silencing of the XAF1 gene by promoter hypermethylation in cancer cells and reactivation to TRAIL-sensitization by IFN-β. BMC Cancer 2007; 7: 52.
Leaman DW, Chawla-Sarkar M, Vyas K, Reheman M, Tamai K, Toji S et al. Identification of X-linked inhibitor of apoptosis-associated factor-1 as an interferon-stimulated gene that augments TRAIL Apo2L-induced apoptosis. J Biol Chem 2002; 277: 28504–28511.
Stein GH, Drullinger LF, Soulard A, Dulic V . Differential roles for cyclin-dependent kinase inhibitors p21 and p16 in the mechanisms of senescence and differentiation in human fibroblasts. Mol Cell Biol 1999; 19: 2109–2117.
Collins CJ, Sedivy JM . Involvement of the INK4a/Arf gene locus in senescence. Aging Cell 2003; 2: 145–150.
Fang L, Igarashi M, Leung J, Sugrue MM, Lee SW, Aaronson SA . p21Waf1/Cip1/Sdi1 induces permanent growth arrest with markers of replicative senescence in human tumor cells lacking functional p53. Oncogene 1999; 18: 2789–2797.
McConnell BB, Starborg M, Brookes S, Peters G . Inhibitors of cyclin-dependent kinases induce features of replicative senescence in early passage human diploid fibroblasts. Curr Biol 1998; 8: 351–354.
Kawasaki T, Tomita Y, Bilim V, Takeda M, Takahashi K, Kumanishi T . Abrogation of apoptosis induced by DNA-damaging agents in human bladder-cancer cell lines with p21/WAF1/CIP1 and/or p53 gene alterations. Int J Cancer 1996; 68: 501–505.
Waldman T, Kinzler KW, Vogelstein B . P21 is necessary for the p53-mediated G1 arrest in human cancer cells. Cancer Res 1995; 55: 5187–5190.
Kosar M, Bartkova J, Hubackova S, Hodny Z, Lukas J, Bartek J . Senescence-associated heterochromatin foci are dispensable for cellular senescence, occur in a cell type- and insult-dependent manner and follow expression of p16(ink4a). Cell Cycle 2011; 10: 457–468.
Narita M, Krizhanovsky V, Nunez S, Chicas A, Hearn SA, Myers MP et al. A novel role for high-mobility group a proteins in cellular senescence and heterochromatin formation. Cell 2006; 126: 503–514.
Takaoka A, Hayakawa S, Yanai H, Stoiber D, Negishi H, Kikuchi H et al. Integration of interferon-alpha/beta signalling to p53 responses in tumour suppression and antiviral defence. Nature 2003; 424: 516–523.
Munoz-Fontela C, Macip S, Martinez-Sobrido L, Brown L, Ashour J, Garcia-Sastre A et al. Transcriptional role of p53 in interferon-mediated antiviral immunity. J Exp Med 2008; 205: 1929–1938.
Zou B, Chim CS, Pang R, Zeng H, Dai Y, Zhang RX et al. XIAP-associated factor 1 (XAF1), a novel target of p53, enhances p53-mediated apoptosis via post-translational modification. Mol Carcinogen 2012; 51: 422–432.
Gong JG, Ammanamanchi S, Ko TC, Brattain MG . Transforming growth factor beta 1 increases the stability of p21/WAF1/CIP1 protein and inhibits CDK2 kinase activity in human colon carcinoma FET cells. Cancer Res 2003; 63: 3340–3346.
Beck SE, Jung BH, Del Rosario E, Gomez J, Carethers JM . BMP-induced growth suppression in colon cancer cells is mediated by p21 (WAF1) stabilization and modulated by RAS/ERK. Cell Signal 2007; 19: 1465–1472.
Fan YM, Chen H, Qiao B, Liu ZW, Luo L, Wu YF et al. c-Jun NH2-terminal kinase decreases ubiquitination and promotes stabilization of p21 (WAF1/CIP1) in K562 cell. Biochem Biophys Res Commun 2007; 355: 263–268.
Abbas T, Dutta A . P21 in cancer: intricate networks and multiple activities. Nat Rev Cancer 2009; 9: 400–414.
Roninson IB . Oncogenic functions of tumour suppressor p21 (Waf1/Cip1/Sdi1): association with cell senescence and tumour-promoting activities of stromal fibroblasts. Cancer Lett 2002; 179: 1–14.
Jascur T, Brickner H, Salles-Passador I, Barbier V, El Khissiin A, Smith B et al. Regulation of p21 (WAF1/Cip1) stability by Wisp39, a Hsp90 binding TPR protein. Mol Cell 2005; 17: 237–249.
Kim Y, Starostina NG, Kipreos ET . The CRL4 (Cdt2) ubiquitin ligase targets the degradation of p21(Cip1) to control replication licensing. Genes Dev 2008; 22: 2507–2519.
Amador V, Ge S, Santamaría PG, Guardavaccaro D, Pagano M . APC/C (Cdc20) controls the ubiquitin-mediated degradation of p21 in prometaphase. Mol Cell 2007; 27: 462–473.
Frescas D, Pagano M . Deregulated proteolysis by the F-box proteins SKP2 and beta-TrCP: tipping the scales of cancer. Nat Rev Cancer 2008; 8: 438–449.
Ueki T, Nishidate T, Park JH, Lin ML, Shimo A, Hirata K et al. Involvement of elevated expression of multiple cell-cycle regulator, DTL/RAMP (denticleless/RA-regulated nuclear matrix associated protein), in the growth of breast cancer cells. Oncogene 2008; 27: 5672–5683.
Yasui K, Arii S, Zhao C, Imoto I, Ueda M, Nagai H et al. TFDP1, CUL4A, and CDC16 identified as targets for amplification at 13q34 in hepatocellular carcinomas. Hepatology 2002; 35: 1476–1484.
Fan BA, Dachrut S, Coral H, Yuen ST, Chu KM, Law S et al. Integration of DNA copy number alterations and transcriptional expression analysis in human gastric cancer. PLoS One 2012; 7: e29824.
Sakakura C, Hagiwara A, Yasuoka R, Fujita Y, Nakanishi M, Masuda K et al. Amplification and over-expression of the AIB1 nuclear receptor co-activator gene in primary gastric cancers. Int J Cancer 2000; 89: 217–223.
van Dekken H, Vissers K, Tilanus HW, Kuo WL, Tanke HJ, Rosenberg C et al. Genomic array and expression analysis of frequent high-level amplifications in adenocarcinomas of the gastro-esophageal junction. Cancer Genet Cytogen 2006; 166: 157–162.
Yoon S, Choi MH, Chang MS, Baik JH . Wnt5a-dopamine D2 receptor interactions regulate dopamine neuron development via extracellular signal-regulated kinase (ERK) activation. J Biol Chem 2011; 286: 15641–15651.
Acknowledgements
For expert technical assistance and helpful discussions we are grateful to Jae-Sung Yi, Chang-Seok Lee and Young-Gyu Ko (Korea University, Seoul, South Korea). This work was supported in part by grants to SGC (2009-0078864 and 2009-0087099) and MGL (353-2009-2-C00071) from National Research Foundation of Korea.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by J-C Marine
Author contributions
JH designed the study, performed the experiments and analyzed the data; YK, KWL, NGH, SIJ and JHL carried out molecular works; MJK and BKR analyzed human cancers; TKH performed statistical analyses; SY and JHB investigated brain development and designed a part of the study. SGC and MGL obtained funding, designed the study and wrote the manuscript.
Supplementary Information accompanies this paper on Cell Death and Differentiation website
Supplementary information
Rights and permissions
About this article
Cite this article
Han, J., Kim, YL., Lee, KW. et al. ZNF313 is a novel cell cycle activator with an E3 ligase activity inhibiting cellular senescence by destabilizing p21WAF1. Cell Death Differ 20, 1055–1067 (2013). https://doi.org/10.1038/cdd.2013.33
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cdd.2013.33
Keywords
This article is cited by
-
XAF1 drives apoptotic switch of endoplasmic reticulum stress response through destabilization of GRP78 and CHIP
Cell Death & Disease (2022)
-
XAF1 destabilizes estrogen receptor α through the assembly of a BRCA1-mediated destruction complex and promotes estrogen-induced apoptosis
Oncogene (2022)
-
RING-finger protein 166 plays a novel pro-apoptotic role in neurotoxin-induced neurodegeneration via ubiquitination of XIAP
Cell Death & Disease (2020)
-
Harnessing the anti-cancer natural product nimbolide for targeted protein degradation
Nature Chemical Biology (2019)
-
The UbL-UBA Ubiquilin4 protein functions as a tumor suppressor in gastric cancer by p53-dependent and p53-independent regulation of p21
Cell Death & Differentiation (2019)