Abstract
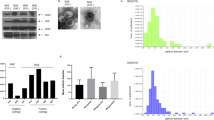
Ovarian cancers are very aggressive cancers most often diagnosed when metastasis has already occurred in the entire peritoneal cavity. Ovarian adenocarcinoma cells present an undetectable level of RhoB GTPase. Using preclinical ovarian cancer models, we aimed to evaluate the potential use of RhoB cDNA as a tumor suppressor gene in gene therapy. RhoB restoration in vitro, through recombinant adenovirus transduction, resulted in the apoptosis of endogenous RhoB protein low-expressing cell lines (OVCAR-3 and IGROV-1) through the activation of the intrinsic apoptotic caspase cascade. We showed that a single injection of 108 p.f.u. of adenoviral vector encoding a reporter gene into the peritoneal cavity of ovarian tumor bearing mice can induce the gene modification of a large quantity of cells throughout the cavity. We thereby tested the effect of AdRhoB injections to treat ovarian cancer-bearing mice. The ectopic expression of RhoB, following its introduction via viral transduction into nude mice in vivo, was highly effective in suppressing tumor growth of ovarian cancer xenografts. Therapeutic agents designed to correct defects of RhoB at the molecular level may thereby provide innovative treatment options for patients not responding to standard therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Abbreviations
- Ad:
-
adenovirus
- CMV:
-
cytomegalovirus
- FCS:
-
fetal calf serum
- GFP:
-
green fluorescent protein
- MOI:
-
multiplicity of infection
- OVCAR-3:
-
NIH-OVCAR3 cells
- RhoB:
-
Ras homologous gene B
References
Wang E, Ngalame Y, Panelli MC, Nguyen-Jackson H, Deavers M, Mueller P et al. Peritoneal and subperitoneal stroma may facilitate regional spread of ovarian cancer. Clin Cancer Res 2005; 11: 113–122.
Symons M, Rusk N . Control of vesicular trafficking by Rho GTPases. Curr Biol 2003; 13: R409–R418.
Chang F, Steelman LS, Lee JT, Shelton JG, Navolanic PM, Blalock WL et al. Signal transduction mediated by the Ras/Raf/MEK/ERK pathway from cytokine receptors to transcription factors: potential targeting for therapeutic intervention. Leukemia 2003; 17: 1263–1293.
Ridley AJ . Rho proteins and cancer. Breast Cancer Res Treat 2004; 84: 13–19.
Huang M, Prendergast GC . RhoB in cancer suppression. Histol Histopathol 2006; 21: 213–218.
Chen Z, Sun J, Pradines A, Favre G, Adnane J, Sebti SM . Both farnesylated and geranylgeranylated RhoB inhibit malignant transformation and suppress human tumor growth in nude mice. J Biol Chem 2000; 275: 17974–17978.
Mazieres J, Antonia T, Daste G, Muro-Cacho C, Berchery D, Tillement V et al. Loss of RhoB expression in human lung cancer progression. Clin Cancer Res 2004; 10: 2742–2750.
Mazieres J, Tillement V, Allal C, Clanet C, Bobin L, Chen Z et al. Geranylgeranylated, but not farnesylated, RhoB suppresses Ras transformation of NIH-3T3 cells. Exp Cell Res 2005; 304: 354–364.
Liu AX, Rane N, Liu JP, Prendergast GC . RhoB is dispensable for mouse development, but it modifies susceptibility to tumor formation as well as cell adhesion and growth factor signaling in transformed cells. Mol Cell Biol 2001; 21: 6906–6912.
Fritz G, Kaina B . Ras-related GTPase RhoB forces alkylation-induced apoptotic cell death. Biochem Biophys Res Commun 2000; 268: 784–789.
Liu A, Du W, Liu JP, Jessell TM, Prendergast GC . RhoB alteration is necessary for apoptotic and antineoplastic responses to farnesyltransferase inhibitors. Mol Cell Biol 2000; 20: 6105–6113.
Liu A, Cerniglia GJ, Bernhard EJ, Prendergast GC . RhoB is required to mediate apoptosis in neoplastically transformed cells after DNA damage. Proc Natl Acad Sci USA 2001; 98: 6192–6197.
Jiang K, Sun J, Cheng J, Djeu JY, Wei S, Sebti S . Akt mediates Ras downregulation of RhoB, a suppressor of transformation, invasion, and metastasis. Mol Cell Biol 2004; 24: 5565–5576.
Wang S, Yan-Neale Y, Fischer D, Zeremski M, Cai R, Zhu J et al. Histone deacetylase 1 represses the small GTPase RhoB expression in human nonsmall lung carcinoma cell line. Oncogene 2003; 22: 6204–6213.
Sato J, Iiri T . [Rho and Rho-kinase]. Nippon Rinsho 2006; 64 (Suppl 5): 197–202.
Adnane J, Muro-Cacho C, Mathews L, Sebti SM, Munoz-Antonia T . Suppression of rho B expression in invasive carcinoma from head and neck cancer patients. Clin Cancer Res 2002; 8: 2225–2232.
Forget MA, Desrosiers RR, Del M, Moumdjian R, Shedid D, Berthelet F et al. The expression of rho proteins decreases with human brain tumor progression: potential tumor markers. Clin Exp Metastasis 2002; 19: 9–15.
Wolf JK, Bodurka DC, Gano JB, Deavers M, Ramondetta L, Ramirez PT et al. A phase I study of Adp53 (INGN 201; ADVEXIN) for patients with platinum- and paclitaxel-resistant epithelial ovarian cancer. Gynecol Oncol 2004; 94: 442–448.
Fabbri M, Iliopoulos D, Trapasso F, Aqeilan RI, Cimmino A, Zanesi N et al. WWOX gene restoration prevents lung cancer growth in vitro and in vivo. Proc Natl Acad Sci USA 2005; 102: 15611–15616.
Pantel K, Cote RJ, Fodstad O . Detection and clinical importance of micrometastatic disease. J Natl Cancer Inst 1999; 91: 1113–1124.
Delord JP, Allal C, Canal M, Mery E, Rochaix P, Hennebelle I et al. Selective inhibition of HER2 inhibits AKT signal transduction and prolongs disease-free survival in a micrometastasis model of ovarian carcinoma. Ann Oncol 2005; 16: 1889–1897.
Eisenhauer EA, Vermorken JB, van Glabbeke M . Predictors of response to subsequent chemotherapy in platinum pretreated ovarian cancer: a multivariate analysis of 704 patients (see comments). Ann Oncol 1997; 8: 963–968.
van der Burg ME, van Lent M, Buyse M, Kobierska A, Colombo N, Favalli G et al. The effect of debulking surgery after induction chemotherapy on the prognosis in advanced epithelial ovarian cancer. Gynecological Cancer Cooperative Group of the European Organization for Research and Treatment of Cancer. N Engl J Med 1995; 332: 629–634.
Zeimet AG, Marth C . Why did p53 gene therapy fail in ovarian cancer? Lancet Oncol 2003; 4: 415–422.
Quist SR, Wang-Gohrke S, Kohler T, Kreienberg R, Runnebaum IB . Cooperative effect of adenoviral p53 gene therapy and standard chemotherapy in ovarian cancer cells independent of the endogenous p53 status. Cancer Gene Ther 2004; 11: 547–554.
Koester SK, Bolton WE . Differentiation and assessment of cell death. Clin Chem Lab Med 1999; 37: 311–317.
Guichard S, Montazeri A, Chatelut E, Hennebelle I, Bugat R, Canal P . Schedule-dependent activity of topotecan in OVCAR-3 ovarian carcinoma xenograft: pharmacokinetic and pharmacodynamic evaluation. Clin Cancer Res 2001; 7: 3222–3228.
Wolf BB, Schuler M, Li W, Eggers-Sedlet B, Lee W, Tailor P et al. Defective cytochrome c-dependent caspase activation in ovarian cancer cell lines due to diminished or absent apoptotic protease activating factor-1 activity. J Biol Chem 2001; 276: 34244–34251.
Aunoble B, Sanches R, Didier E, Bignon YJ . Major oncogenes and tumor suppressor genes involved in epithelial ovarian cancer (review). Int J Oncol 2000; 16: 567–576.
Chaigne-Delalande B, Deuve L, Reuzeau E, Basoni C, Lafarge D, Varon C et al. RhoGTPases and p53 are involved in the morphological appearance and interferon-alpha response of hairy cells. Am J Pathol 2006; 168: 562–573.
Vasey PA, Shulman LN, Campos S, Davis J, Gore M, Johnston S et al. Phase I trial of intraperitoneal injection of the E1B-55-kd-gene-deleted adenovirus ONYX-015 (dl1520) given on days 1 through 5 every 3 weeks in patients with recurrent/refractory epithelial ovarian cancer. J Clin Oncol 2002; 20: 1562–1569.
Bauerschmitz GJ, Lam JT, Kanerva A, Suzuki K, Nettelbeck DM, Dmitriev I et al. Treatment of ovarian cancer with a tropism modified oncolytic adenovirus. Cancer Res 2002; 62: 1266–1270.
McNeish IA, Bell SJ, Lemoine NR . Gene therapy progress and prospects: cancer gene therapy using tumour suppressor genes. Gene Therapy 2004; 11: 497–503.
Acknowledgements
The fluorescence imaging set up was partly supported by the IPA CNRS CEA program. We are grateful to Lourdes Gasquet and Christiane Pages at the animal facility. This study was supported by grants from The Region Midi Pyrénées, the GRICR (Groupe de Recherche Institut Claudius Regaud) and the University Toulouse III (France). Viral vectors have been produced in the Institut Claudius Regaud's vector core granted by the ‘ligue contre le cancer’.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Couderc, B., Pradines, A., Rafii, A. et al. In vivo restoration of RhoB expression leads to ovarian tumor regression. Cancer Gene Ther 15, 456–464 (2008). https://doi.org/10.1038/cgt.2008.12
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/cgt.2008.12
Keywords
This article is cited by
-
RhoB expression associated with chemotherapy response and prognosis in colorectal cancer
Cancer Cell International (2024)
-
SDF-1alpha concentration dependent modulation of RhoA and Rac1 modifies breast cancer and stromal cells interaction
BMC Cancer (2015)
-
Mesenchymal cell interaction with ovarian cancer cells induces a background dependent pro-metastatic transcriptomic profile
Journal of Translational Medicine (2014)
-
NSC126188 induces apoptosis of prostate cancer PC-3 cells through inhibition of Akt membrane translocation, FoxO3a activation, and RhoB transcription
Apoptosis (2014)
-
RhoB modifies estrogen responses in breast cancer cells by influencing expression of the estrogen receptor
Breast Cancer Research (2013)