Abstract
Purpose
To describe the use of the second-generation QuantiFERON-TB Gold (QFT-G) test in a series of patients in an ophthalmic practice.
Methods
The charts of all patients who had QFT-G tests ordered by Mayo Clinic ophthalmologists in the past 3 years were reviewed.
Results
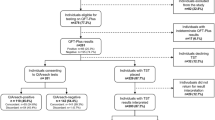
A total of 27 QFT-G tests were ordered. Thirteen (48%) tests were negative, six (22%) were indeterminate, two (7%) tests were re-ordered after a lab accident or an improper cancellation, four (15%) were positive and represented infection, and two (7%) were positive but negative when re-tested. Of the four truly positive cases, three were treated for tuberculosis (TB): one had tuberculous iritis, one had retinal vasculitis and haemorrhage, and one had asymptomatic TB but was on immunosuppressive therapy. The fourth patient had previously been treated for latent infection.
Conclusions
In a series of selected patients with uveitis, the QFT-G test was able to detect TB infection in 15% of the patients, though it does not differentiate between active and latent TB infection. QFT-G should be considered in place of purified protein derivative testing in those with uveitis that have had prior BCG vaccination and in immunocompromised patients. Patients with a positive QFT-G, but who have little risk for TB infection and a negative systemic work-up, should be re-tested.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Thompson MJ, Albert DM . Ocular tuberculosis. Arch Ophthalmol 2005; 123: 844–849.
Ferrara G, Losi M, Meacci M, Meccugni B, Piro R, Roversi P et al Routine hospital use of a new commercial whole blood interferon-gamma assay for the diagnosis of tuberculosis infection. Am J Respir Crit Care Med 2005; 172: 631–635.
Mazurek GH, Jereb J, Lobue P, Iademarco MF, Metchock B, Vernon A . Guidelines for using the QuantiFERON-TB Gold test for detecting Mycobacterium tuberculosis infection, United States. MMWR Recomm Rep 2005; 54: 49–55.
Mori T, Sakatani M, Yamagishi F, Takashima T, Kawabe Y, Nagao K et al Specific detection of tuberculosis infection: an interferon-gamma-based assay using new antigens. Am J Respir Crit Care Med 2004; 170: 59–64.
Menzies D . Interpretation of repeated tuberculin tests. Boosting, conversion, and reversion. Am J Respir Crit Care Med 1999; 159: 15–21.
Jones S, de Gijsel D, Wallach FR, Gurtman AC, Shi Q, Sacks H. Utility of QuantiFERON-TB Gold in-tube testing for latent TB infection in HIV-infected individuals. Int J Tuberc Lund Dis 2007; 11: 1190–1195.
Mazurek GH, LoBue PA, Daley CL, Bernardo J, Lardizabal AA, Bishai WR et al Comparison of a whole-blood interferon gamma assay with tuberculin skin testing for detecting latent Mycobacterium tuberculosis infection. JAMA 2001; 286: 1740–1747.
Kobashi Y, Mouri K, Obase Y, Fukuda M, Miyashita N, Oka M . Clinical evaluation of QuantiFERON TB-2G test for immunocompromised patients. Eur Respir J 2007; 30: 945–950.
Pai M, Joshi R, Dogra S, Mendiratta DK, Narang P, Kalantri S et al Serial testing of health care workers for tuberculosis using interferon-gamma assay. Am J Respir Crit Care Med 2006; 174: 349–355.
Acknowledgements
We thank Elaine Beito and Dr Robin Molella for their helpful comments. This study was supported by an unrestricted grant from Research to Prevent Blindness Inc., New York, NY and the Mayo Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors indicate no financial conflict of interest.
Contributions of authors: Involved in the design and conduct of the study, collection, management, analysis and interpretation of data, and preparation, review and approval of the manuscript and references (SI, SJB, JSP); involved in the design and conduct of the study (DCH, LJF, GTT, SRB, NSF); The study protocol was approved by the Mayo Institutional Review Board and conforms to HIPAA requirements. As a retrospective analysis, informed consent was not required from participants. However, in conformity with Minnesota state law, we did not include in this study any patients who have refused to allow their medical records reviewed for the purpose of medical research.
Supplementary Information accompanies the paper on Eye website (http://www.nature.com/eye)
Supplementary information
Rights and permissions
About this article
Cite this article
Itty, S., Bakri, S., Pulido, J. et al. Initial results of QuantiFERON-TB Gold testing in patients with uveitis. Eye 23, 904–909 (2009). https://doi.org/10.1038/eye.2008.115
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/eye.2008.115
Keywords
This article is cited by
-
Presumed ocular tuberculosis in the United Kingdom: a British Ophthalmological Surveillance Unit (BOSU) study
Eye (2020)
-
Structural changes of the choroid in sarcoid- and tuberculosis-related granulomatous uveitis
Eye (2015)
-
Clinical presentation, treatment, and outcomes in presumed intraocular tuberculosis: experience from Newcastle upon Tyne, UK
Eye (2013)
-
Utility of QuantiFERON®-TB Gold test in diagnosis and management of suspected tubercular uveitis in India
International Ophthalmology (2012)
-
The value of an immune response to Mycobacterium tuberculosis in patients with chronic posterior uveitides revisited: utility of the new IGRAs
Eye (2010)