Abstract
Purpose
The purpose of this study was to report the 2-year outcome of an individually tailored ‘observe-and-plan’ treatment regimen for neovascular age-related macular degeneration (nAMD), and to investigate its clinical value in terms of functional outcome. This regimen aimed to reduce the clinical burden (visits) by employing individually fixed injection intervals, based on the predictability of an individual’s need for retreatment.
Methods
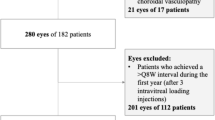
This prospective case series included 104 patients (115 eyes) with nAMD. Following three loading doses of ranibizumab, the disease recurrence interval was determined in monthly observation visits. Retreatment was applied in a series of three injections with individually fixed intervals (2 weeks shorter than the recurrence interval), combined with periodic adjustment of the intervals. The allowed injection intervals in treatment plans ranged from 1 to 3 months. If there was no recurrence at 3 months, the patient could change to monitoring alone.
Results
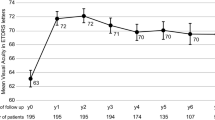
Mean visual acuity (VA) improved by 8.7, 9.7, and 9.2 letters at months 3, 12, and 24, respectively. The mean number of injections was 7.8 and 5.8 during years 1 and 2, respectively, whereas the mean number of ophthalmic examinations was 4.0 and 2.9, respectively. The mean treatment interval (after the loading doses) was 2.0 months during year 1, and 2.2 months during year 2.
Conclusion
The observe-and-plan regimen significantly improved and maintained VA over the course of 2 years. This favourable functional outcome was achieved with fewer clinic visits compared with other regimens. Therefore, this observe-and-plan regimen has the potential to alleviate the clinical burden of nAMD treatment.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Change history
12 March 2015
This article has been corrected since advance online publication and a corrigendum is also printed in this issue
References
Resnikoff S, Pascolini D, Etya'ale D, Kocur I, Pararajasegaram R, Pokharel GP et al. Global data on visual impairment in the year 2002. Bull World Health Organ 2004; 82 (11): 844–851.
Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 2006; 355 (14): 1432–1444.
Brown DM, Michels M, Kaiser PK, Heier JS, Sy JP, Ianchulev T . Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: two-year results of the ANCHOR study. Ophthalmology 2009; 116 (1): 57–65.
Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006; 355 (14): 1419–1431.
Schmidt-Erfurth U, Kaiser PK, Korobelnik JF, Brown DM, Chong V, Nguyen QD et al. Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six-week results of the VIEW studies. Ophthalmology 2014; 121 (1): 193–201.
Heier JS, Brown DM, Chong V, Korobelnik JF, Kaiser PK, Nguyen QD et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology 2012; 119 (12): 2537–2548.
Bloch SB, Larsen M, Munch IC . Incidence of legal blindness from age-related macular degeneration in Denmark: year 2000 to 2010. Am J Ophthalmol 2012; 153 (2): 209–213 e202.
Abraham P, Yue H, Wilson L . Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER study year 2. Am J Ophthalmol 2010; 150 (3): 315–324.
Regillo CD, Brown DM, Abraham P, Yue H, Ianchulev T, Schneider S et al. Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER Study year 1. Am J Ophthalmol 2008; 145 (2): 239–248.
Schmidt-Erfurth U, Eldem B, Guymer R, Korobelnik JF, Schlingemann RO, xer-Siegel R et al. Efficacy and safety of monthly versus quarterly ranibizumab treatment in neovascular age-related macular degeneration: the EXCITE study. Ophthalmology 2011; 118 (5): 831–839.
Busbee BG, Ho AC, Brown DM, Heier JS, Suner IJ, Li Z et al. Twelve-month efficacy and safety of 0.5mg or 2.0mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology 2013; 120 (5): 1046–1056.
Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ . Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med 2011; 364 (20): 1897–1908.
Han DP . Age-related macular degeneration, anti-VEGF therapy, and ophthalmic imaging: is there a best practice? JAMA Ophthalmol 2013; 131 (9): 1124–1126.
Mantel I, Niderprim SA, Gianniou C, Deli A, Ambresin A . Reducing the clinical burden of ranibizumab treatment for neovascular age-related macular degeneration using an individually planned regimen. Br J Ophthalmol 2014; 98 (9): 1192–1196.
Mantel I, Deli A, Iglesias K, Ambresin A . Prospective study evaluating the predictability of need for retreatment with intravitreal ranibizumab for age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 2013; 251 (3): 697–704.
IVAN Study Investigators Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ et al. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology 2012; 119 (7): 1399–1411.
Fung AE, Lalwani GA, Rosenfeld PJ, Dubovy SR, Michels S, Feuer WJ et al. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol 2007; 143 (4): 566–583.
Gupta OP, Shienbaum G, Patel AH, Fecarotta C, Kaiser RS, Regillo CD . A treat and extend regimen using ranibizumab for neovascular age-related macular degeneration clinical and economic impact. Ophthalmology 2010; 117 (11): 2134–2140.
Haller JA . Current anti-vascular endothelial growth factor dosing regimens: benefits and burden. Ophthalmology 2013; 120 (5 Suppl): S3–S7.
Lalwani GA, Rosenfeld PJ, Fung AE, Dubovy SR, Michels S, Feuer W et al. A variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: year 2 of the PrONTO Study. Am J Ophthalmol 2009; 148 (1): 43–58.
Holz FG, Amoaku W, Donate J, Guymer RH, Kellner U, Schlingemann RO et al. Safety and efficacy of a flexible dosing regimen of ranibizumab in neovascular age-related macular degeneration: the SUSTAIN study. Ophthalmology 2011; 118 (4): 663–671.
Tschuor P, Pilly B, Venugopal D, Gale RP . Optimising assessment intervals improves visual outcomes in ranibizumab-treated age-related neovascular degeneration: using the stability phase as a benchmark. Graefes Arch Clin Exp Ophthalmol 2013; 251 (10): 2327–2330.
Wolf A, Kampik A . Efficacy of treatment with ranibizumab in patients with wet age-related macular degeneration in routine clinical care: data from the COMPASS health services research. Graefes Arch Clin Exp Ophthalmol 2014; 252 (4): 647–655.
Dadgostar H, Ventura AA, Chung JY, Sharma S, Kaiser PK . Evaluation of injection frequency and visual acuity outcomes for ranibizumab monotherapy in exudative age-related macular degeneration. Ophthalmology 2009; 116 (9): 1740–1747.
Gerding H . [Treatment of wet AMD with less than 12 injections of Ranibizumab per year]. Klin Monatsbl Augenheilkd 2010; 227 (4): 294–297.
Gerding H . Ranibizumab treatment in age-related macular degeneration: a meta-analysis of one-year results. Klin Monatsbl Augenheilkd 2014; 231 (4): 427–431.
Writing Committee for the UK Age-Related Macular Degeneration EMR Users Group. The neovascular age-related macular degeneration database: multicenter study of 92 976 ranibizumab injections: report 1: visual acuity. Ophthalmology 2014; 121 (5): 1092–1101.
Rosenfeld PJ, Shapiro H, Tuomi L, Webster M, Elledge J, Blodi B et al. Characteristics of patients losing vision after 2 years of monthly dosing in the phase III ranibizumab clinical trials. Ophthalmology 2011; 118 (3): 523–530.
Acknowledgements
We thank Mrs Mary C Love for her editorial help.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Eye website
Supplementary information
Rights and permissions
About this article
Cite this article
Gianniou, C., Dirani, A., Ferrini, W. et al. Two-year outcome of an observe-and-plan regimen for neovascular age-related macular degeneration: how to alleviate the clinical burden with maintained functional results. Eye 29, 342–349 (2015). https://doi.org/10.1038/eye.2014.258
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/eye.2014.258
This article is cited by
-
Delaying anti-VEGF therapy during the COVID-19 pandemic: long-term impact on visual outcomes in patients with neovascular age-related macular degeneration
BMC Ophthalmology (2023)
-
A model to quantify the influence of treatment patterns and optimize outcomes in nAMD
Scientific Reports (2022)
-
Modifiable Determinants of Satisfaction with Intravitreal Treatment in Patients with Neovascular Age-Related Macular Degeneration
Drugs & Aging (2022)
-
Erste Erfahrungen mit Brolucizumab bei neovaskulärer altersabhängiger Makuladegeneration und Therapierefraktärität unter der bisherigen Anti-VEGF-Therapie
Der Ophthalmologe (2022)
-
Refractory neovascular age-related macular degeneration: time-dependent changes of central retinal thickness with anti-VEGF treatment
Graefe's Archive for Clinical and Experimental Ophthalmology (2021)