Abstract
Purpose
Intraocular vascular endothelial growth factor (VEGF) levels increases with the severity of diabetic retinopathy. Response of diabetic macular oedema (DMO) to ranibizumab is driven by VEGF suppression. We hypothesised that the initial reduction of central macular thickness by ranibizumab should be maximum in severe diabetic retinopathy until the levels of VEGF decreases to the levels observed in eyes with mild retinopathy.
Methods
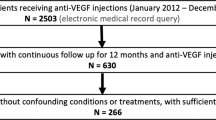
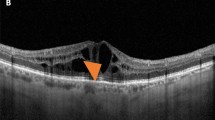
Consecutive patients with centre-involving DMO (central subfield thickness (CSFT)>300 μm) who had three consecutive monthly ranibizumab injections followed by as needed therapy were included. Retinopathy status was graded as mild non-proliferative diabetic retinopathy (NPDR) (G1), moderate to severe NPDR with no prior panretinal photocoagulation (G2), and treated PDR (G3).
Results
Two hundred and thirty-nine eyes from 204 patients with a mean age of 64.9 years were included. The distribution was 31.4 G1, 32.2 G2, and 36.4% G3. Mean baseline CSFT for all eyes was 458.5±110.8 μm. Baseline CSFT for G1, G2, and G3, respectively, were 437.6±90.9, 472.3±109.8, and 464.7±124.9 μm (P=0.2155). Mean change in CSFT after three consecutive injections was 128.5±116.6 μm. The mean changes were 95.8±101.4 μm for G1, 137.2±112.9 μm for G2, and 148.9±126.9 μm for G3. The changes in CSFT between groups adjusted for baseline CSFT were statistically significant (P=0.0473). At 6 and 12 months after a mean of 4.5 and 7.7 injections, the changes between groups were no longer significant, P=0.4783 and P=0.8271, respectively.
Conclusions
The initial anatomical response of DMO with intravitreal ranibizumab injections was maximum in eyes with treated PDR, suggesting that the higher the VEGF levels, the better the response with ranibizumab.
Similar content being viewed by others
Log in or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Antcliff RJ, Marshall J . The pathogenesis of edema in diabetic maculopathy. Semin Ophthalmol 1999; 14: 223–232.
Funatsu H, Yamashita H, Sakata K, Noma H, Mimura T, Suzuki M et al. Vitreous levels of vascular endothelial growth factor and intercellular adhesion molecule 1 are related to diabetic macular edema. Ophthalmology 2005; 112: 806–816.
Nguyen QD, Shah SM, Khwaja AA, Channa R, Hatef E, Do DV et al. Two-year outcomes of the ranibizumab for edema of the mAcula in diabetes (READ-2) study. Ophthalmology 2010; 117: 2146–2151.
Comyn O, Sivaprasad S, Peto T, Neveu MM, Holder GE, Xing W et al. A randomized trial to assess functional and structural effects of ranibizumab versus laser in diabetic macular edema (the LUCIDATE study. Am J Ophthalmol 2014; 157: 960–970.
Mitchell P, Bandello F, Schmidt-Erfurth U, Lang GE, Massin P, Schlingemann RO et al. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology 2011; 118: 615–625.
Massin P, Bandello F, Garweg JG, Hansen LL, Harding SP, Larsen M et al. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): a 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care 2010; 33: 2399–2405.
Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med 1994; 331: 1480–1487.
Watanabe D, Suzuma K, Suzuma I, Ohashi H, Ojima T, Kurimoto M et al. Vitreous levels of angiopoietin 2 and vascular endothelial growth factor in patients with proliferative diabetic retinopathy. Am J Ophthalmol 2005; 139: 476–481.
Funatsu H, Yamashita H, Noma H, Mimura T, Nakamura S, Sakata K et al. Aqueous humor levels of cytokines are related to vitreous levels and progression of diabetic retinopathy in diabetic patients. Graefes Arch Clin Exp Ophthalmol 2005; 243: 3–8.
Arimura S, Takamura Y, Miyake S, Gozawa M, Iwasaki K, Tomomatsu T et al. The effect of triamcinolone acetonide or bevacizumab on the levels of proinflammatory cytokines after retinal laser photocoagulation in pigmented rabbits. Exp Eye Res 2016; 149: 1–7.
Bressler SB, Qin H, Beck RW, Chalam KV, Kim JE, Melia M et al. Factors associated with changes in visual acuity and central subfield thickness at 1 year after treatment for diabetic macular edema with ranibizumab. Arch Ophthalmol 1960 2012; 130: 1153–1161.
Ferris FL, Miller KM, Glassman AR, Beck RW,, Diabetic Retinopathy Clinical Research Network. A proposed method of logarithmic transformation of optical coherence tomography data for use in clinical research. Ophthalmology 2010; 117: 1512–1516.
Brown DM, Campochiaro PA, Singh RP, Li Z, Gray S, Saroj N et al. Ranibizumab for macular edema following central retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology 2010; 117: 1124–1133. e1.
Weiter JJ, Zuckerman R . The influence of the photoreceptor–RPE complex on the inner retina. An explanation for the beneficial effects of photocoagulation. Ophthalmology 1980; 87: 1133–1139.
Stefansson E, Landers MB, Wolbarsht ML . Increased retinal oxygen supply following pan-retinal photocoagulation and vitrectomy and lensectomy. Trans Am Ophthalmol Soc 1981; 79: 307–334.
Stefánsson E, Hatchell DL, Fisher BL, Sutherland FS, Machemer R . Panretinal photocoagulation and retinal oxygenation in normal and diabetic cats. Am J Ophthalmol 1986; 101: 657–664.
Funatsu H, Wilson CA, Berkowitz BA, Sonkin PL . A comparative study of the effects of argon and diode laser photocoagulation on retinal oxygenation. Graefes Arch Clin Exp Ophthalmol 1997; 235: 168–175.
Adamis AP, Shima DT, Yeo KT, Yeo TK, Brown LF, Berse B et al. Synthesis and secretion of vascular permeability factor/vascular endothelial growth factor by human retinal pigment epithelial cells. Biochem Biophys Res Commun 1993; 193: 631–638.
Shima DT, Adamis AP, Ferrara N, Yeo KT, Yeo TK, Allende R et al. Hypoxic induction of endothelial cell growth factors in retinal cells: identification and characterization of vascular endothelial growth factor (VEGF) as the mitogen. Mol Med 1995; 1: 182–193.
Shimura M, Yasuda K, Nakazawa T, Abe T, Shiono T, Iida T et al. Panretinal photocoagulation induces pro-inflammatory cytokines and macular thickening in high-risk proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol 2009; 247: 1617–1624.
Schmidinger G, Maar N, Bolz M, Scholda C, Schmidt-Erfurth U . Repeated intravitreal bevacizumab (Avastin) treatment of persistent new vessels in proliferative diabetic retinopathy after complete panretinal photocoagulation. Acta Ophthalmol 2011; 89: 76–81.
Cintra LP, Costa RA, Ribeiro JA, Calucci D, Scott IU, Messias A et al. Intravitreal bevacizumab (Avastin) for persistent new vessels in diabetic retinopathy (IBEPE study): 1-year results. Retina (Philadelphia, PA) 2013; 33: 1109–1116.
Erdol H, Turk A, Akyol N, Imamoglu HI . The results of intravitreal bevacizumab injections for persistent neovascularizations in proliferative diabetic retinopathy after photocoagulation therapy. Retina (Philadelphia, PA) 2010; 30: 570–577.
Grant MB, Afzal A, Spoerri P, Pan H, Shaw LC, Mames RN . The role of growth factors in the pathogenesis of diabetic retinopathy. Expert Opin Investig Drugs 2004; 13: 1275–1293.
Deissler HL, Deissler H, Lang GE . Inhibition of vascular endothelial growth factor (VEGF) is sufficient to completely restore barrier malfunction induced by growth factors in microvascular retinal endothelial cells. Br J Ophthalmol 2011; 95: 1151–1156.
Nguyen QD, Brown DM, Marcus DM, Boyer DS, Patel S, Feiner L et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology 2012; 119: 789–801.
Holz FG, Roider J, Ogura Y, Korobelnik JF, Simader C, Groetzbach G et al. VEGF Trap-Eye for macular oedema secondary to central retinal vein occlusion: 6-month results of the phase III GALILEO study. Br J Ophthalmol 2013; 97: 278–284.
Diabetic Retinopathy Clinical Research Network Diabetic Retinopathy Clinical Research Network Wells JA, Diabetic Retinopathy Clinical Research Network Glassman AR, Diabetic Retinopathy Clinical Research Network Ayala AR, Diabetic Retinopathy Clinical Research Network Jampol LM, Diabetic Retinopathy Clinical Research Network Aiello LP et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med 2015; 372: 1193–1203.
Campochiaro PA, Brown DM, Awh CC, Lee SY, Gray S, Saroj N et al. Sustained benefits from ranibizumab for macular edema following central retinal vein occlusion: twelve-month outcomes of a phase III study. Ophthalmology 2011; 118: 2041–2049.
Acknowledgements
No funding was received for the work submitted. PGH has received grants from Novartis (Surrey, UK), Allergan (Irvine, CA, USA), and Bayer (Leverkusen, Germany), and is on the advisory board and receives speaker fees from Novartis and Bayer. SS has received grants from Novartis (Surrey, UK), Allergan (Irvine, CA, USA), and Bayer (Leverkusen, Germany), and is on the advisory board and receives speaker fees from Novartis, Allergan, and Bayer. The following authors have no financial disclosures: LN, NVP, JR, CV-A, MM, and RR.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on Eye website
Supplementary information
Rights and permissions
About this article
Cite this article
Nicholson, L., Patrao, N., Ramu, J. et al. Influence of baseline diabetic retinopathy status on initial anatomical response of intravitreal ranibizumab therapy for diabetic macular oedema. Eye 31, 1358–1364 (2017). https://doi.org/10.1038/eye.2017.69
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/eye.2017.69