Abstract
KIR2DS2 is an activating homologue of KIR2DL2, an inhibitory killer-cell immunoglobulin-like receptor (KIR) that surveys expression of major histocompatibility complex-C allotypes bearing a C1 epitope. We have studied here its allele KIR2DS2*005, which shows a hybrid structure—it is identical to other KIR2DS2 alleles in the ectodomain, but has transmembrane and cytoplasmic regions identical to those of KIR2DS3*001, a short-tailed KIR of uncertain expression and function. Our results reveal that KIR2DS2*005 is a fusion gene—the product of an unequal crossing over by which the genes KIR2DS2 and KIR2DS3 recombined within a 400 base pair region of complete identity in intron 6. Also resulting from that recombination was a shortened KIR haplotype of the B group, in which three genes commonly linked to KIR2DS2 (KIR2DL2, KIR2DL5B and KIR2DS3) are deleted. Population studies indicate that KIR2DS2*005 is still associated to such haplotype, and it can be found in approximately 1.2% of Caucasoids. Using a combination of two monoclonal antibodies, we also demonstrate that KIR2DS2*005 encodes a molecule expressed on the surface of natural killer- and T-lymphocytes.
Similar content being viewed by others
Introduction
Killer-cell immunoglobulin-like receptors (KIRs) regulate subsets of natural killer (NK)- and T-lymphocytes upon recognition of major histocompatibility complex (MHC) class-I molecules in target cells.1 Different KIRs have opposite functions that depend on structure: KIRs having long cytoplasmic tails (Cyts) (KIR 2DL- and 3DL-) with immunoreceptor tyrosine-based inhibitory motifs suppress effector functions, whereas KIRs having short Cyts (KIR 2DS− and 3DS−) and a positively charged transmembrane (TM) amino acid activate lymphocytes through an associated DAP12 molecule. The human KIR complex spans a region of 150–200 kbp in the leukocyte receptor complex of chromosome 19 (19q13.4), and comprises 14 KIR genes and 2 pseudogenes.2, 3 The gene content of the KIR complex is extremely variable, which is explained by rapid evolution of KIR by mutation and recombination.4 These processes lead to generation of new genes with extensive allelic polymorphism and copy-number variation in each species.5 Only the genes that mark the centromeric and the telomeric ends of the human KIR complex (KIR3DL3 and KIR3DL2, respectively) are shared by nearly all humans. All other KIR genes appear with different frequencies in each population. A common combination of nine genes and pseudogenes (KIR 3DL3, 2DL3, 2DP1, 2DL1, 3DP1, 2DL4, 3DL1, 2DS4 and 3DL2) is designated as ‘A’ haplotype, any variations in excess from this gene content being considered as ‘B’ haplotype.6
Encoded in the centromeric part of many B haplotypes are the activating receptor KIR2DS2 and its inhibitory homologue KIR2DL2.6 Their genes are consecutive and in nearly complete linkage disequilibrium in Caucasoids. Both KIR2DL2 and its allotype KIR2DL3 recognise HLA-C alleles with asparagine in position 80; in contrast, KIR2DS2 has lost any avidity for HLA-C, despite its ectodomain differing from KIR2DL2 by only three amino-acid substitutions.7 Twenty KIR2DS2 alleles are currently recognised by the Immuno-Polymorphism Database.8 One allele, KIR2DS2*005, has a coding sequence that differs from the common allele KIR2DS2*001 by 10 nucleotide substitutions. These polymorphisms, shared by KIR2DS3*001, concentrate in exons 7–9, and they are translated into one amino-acid change in the TM region and four more in the Cyt region.9
Several aspects of KIR2DS2*005 remain unsolved. First, the hybrid aspect of KIR2DS2*005 could be explained by this allele being the product of a recombination between KIR2DS2 and KIR2DS3*001, but genomic information to support this hypothesis is lacking, and other mechanisms, like convergent evolution after accumulation of successive point mutations, might also account for the features shared by KIR2DS2*005 and KIR2DS3*001. Second, the KIR haplotypes in which KIR2DS2*005 can be found are unknown, as is the distribution of this allele. Third, KIR2DS2*005 gives rise to a correctly processed mRNA, as assessed by isolation of a complete cDNA from a human library;9 however, nothing is known about its translation into a mature protein and its possible expression on the cell surface. Expression is questionable because KIR2DS2*005 has TM and Cyt domains identical to those of KIR2DS3*001, molecule proposed to be retained intracellularly.10 Here, we address these questions through analyses of Caucasoid individuals carrying KIR2DS2*005.
Results
Genetic features of KIR2DS2*005
In a previous study on KIR-B haplotypes, we identified a healthy Caucasoid woman carrying KIR2DS2*005,11 an uncommon allele with a hybrid primary structure. KIR-gene segregation in her sibling revealed that KIR2DS2*005 was inherited in a KIR haplotype that lacked a KIR2DL2 gene (Figure 1), whereas its central and telomeric regions were characteristic of B haplotypes (KIR 3DP1-2DL4-3DS1-2DL5A*001-2DS5-2DS1-3DL2). Independent segregation of KIR2DS2 and KIR2DL2 is rather unusual, particularly among Caucasoids, in whom KIR2DS2 and KIR2DL2 appear in nearly complete linkage disequilibrium.12 An alternative explanation to this phenotype could be the presence of a new KIR2DL2 allele in this family that escaped detection by our genotyping system due to polymorphisms that precluded annealing of polymerase chain reaction (PCR) primers. To rule out this possibility and, also, to map KIR2DS2*005 in the unusual haplotype, we studied its arrangement through analysis of the genes flanking KIR2DS2*005. Using a KIR-gene walking approach,11 we found KIR2DS2*005 downstream of KIR3DL3, the normal arrangement of these two genes. On the contrary, we found that KIR2DS2 was followed not by KIR2DL2 as it happens in most haplotypes, but by the KIR2DP1 pseudogene, from which KIR2DS2 is normally separated by three KIR genes (Figure 1). This finding was in agreement with the lack of KIR2DL2 in the donor, as ascertained by PCR– sequence-specific primer (SSP) typing.
Segregation analysis and KIR-gene walking of KIR2DS2*005 show this allele being inherited within a shortened B haplotype. Parental haplotypes (labelled ‘a’, ‘b’, ‘c’, ‘d’) were deduced from the KIR genotypes of donor C180 and her sibling (shown at the bottom). Using a KIR-gene walking approach in genomic DNA of C180F2, KIR2DS2*005 was mapped downstream of KIR3DL3 and immediately upstream of KIR2DP1; physical linkage in haplotype d, as demonstrated by KIR-gene walking, is represented by triple lines. The KIR2DS2*005-carrying haplotype is compared with a common KIR-B haplotype to illustrate apparent deletion of three genes in the former. Such deletion is consistent with the likely origin of KIR2DS2*005 (Figure 5). KIR3DP1*003 and KIR2DS4*003 were not distinguished from other alleles carrying exon 2 and exon 5 deletions, respectively; and KIR2DS2*001 stands for any KIR2DS2 alleles carrying canonical transmembrane and Cyt regions.
We isolated a cDNA of KIR2DS2*005 from one family member whose genome carried a single copy of the KIR2DS2 gene, and confirmed that its nucleotide sequence matched the coding regions reported previously by Rajalingam et al.9 and Hou et al. (GenBank accession no. EU156177, unpublished). KIR2DS2*005 displays an apparently hybrid primary structure: exons 1 through 5, encoding the extracellular region, are identical to those of other KIR2DS2 alleles; whereas exons 7 through 9, encoding the TM and Cyt regions, match those of a different gene, KIR2DS3*001, and differ from the predominant KIR2DS2*00101 allele by 10 nucleotide substitutions. Exon 6, coding for the stem that connects the Ig-like domains with the TM region, is identical in nearly all alleles of KIR2DS2 (including *005) and of KIR2DS3.
To elucidate whether these hybrid features of KIR2DS2*005 derive from being a fusion gene, or they are the result of convergent evolution (accumulation of successive point mutations in exons 7–9 of KIR2DS2), we amplified 7.7 kbp of KIR2DS2*005 by long-range PCR on genomic DNA of donor C180F2. The amplicon spanned the fifth and seventh exons of KIR2DS2*005, and the intervening introns. Sequence analysis demonstrated (Figure 2): (i) that its 5′ end (down to the 3.2 kbp 5′-most of intron 6) was nearly identical to KIR2DS2*0010102 and differed from KIR2DS3*001 by 33 nucleotide substitutions and two indels; (ii) that, conversely, the 3′-most 650 bp of intron 6 matched almost exactly KIR2DS3*0010301, and differed from KIR2DS2*0010102 by 22 base substitutions and a two-nucleotide indel; and (iii) that the 407 bp in between have the identical sequence in all three alleles. Such pattern of homologies strongly supports that KIR2DS2 and KIR2DS3 recombined at some point within their 407 bp-long identical region in intron 6, producing a fusion gene, KIR2DS2*005, along with a novel KIR haplotype.
Intron 6 sequence supports KIR2DS2*005 being a fusion gene of KIR2DS2 and KIR2DS3. The DNA sequence of KIR2DS2*005 intron 6 is compared with those of KIR2DS2*0010102 and KIR2DS3*0010301 (both from GenBank/EMBL/DDBJ accession no. AY320039). Partial sequences of exons 6 and 7 are shown underlined in capital letters. Numbering refers to the first nucleotide shown in the alignment. Dots represent identity to KIR2DS2*005, and dashes, indels. To reduce figure size, the sequence between nucleotides 800 and 2800 of KIR2DS2*005 has been omitted; in the omitted region, KIR2DS2*005 is identical to KIR2DS2*0010102, and differs from KIR2DS3*0010301 by 22 base substitutions and deletion of one nucleotide. The regions of high homology between KIR2DS2*005 and either KIR2DS2*0010102 or KIR2DS3*0010301 are shaded in the latter two sequences.
Distribution of KIR2DS2*005
Genotyping of KIR2DS2*005 is complicated by the identity of this allele to different other KIR sequences over its 5′- and 3′-exons. Unambiguous PCR identification of its coding sequence would require combining oligonucleotide primers targeting exonic polymorphisms located 7.7 kbp apart. To achieve simpler detection of KIR2DS2*005, we took advantage of the hybrid structure of its sixth intron and designed a PCR–SSP method that combines forward and reverse primers for its KIR2DS2-like and KIR2DS3-like regions, respectively. Those primers amplify specifically a fragment of 669 bp only in KIR2DS2*005-positive DNAs (Figure 3). With this method, we studied 142 KIR2DS2-positive DNAs selected from a population of 248 unrelated Spanish Caucasoids controls. Two individuals, besides cell C180, turned positive, giving a frequency of 1.21% for KIR2DS2*005 (95% confidence interval: 0–2.57%). From one of these donors, C052 (Table 1), we obtained a DNA sequence of KIR2DS2*005 intron 6 that matched exactly the one isolated from family C180.
KIR2DS2*005 is a hybrid gene containing the 5′-end of KIR2DS2 (exon 1–intron 6) and the 3′ end of KIR2DS3 (intron 6–exon 9). Its chimerical structure enables simpler detection of KIR2DS2*005 by PCR–SSP method with primers for intron 6. Two KIR2DS2*005-positive DNAs were tested along with five negative controls. Exons and introns are drawn approximately to scale. IPC, internal positive control of the PCR.
As shown in Table 1, one of the additional KIR2DS2*005 DNAs (C342) shared the KIR phenotype 2DS2pos-(2DL2-2DL5B-2DS3)neg with members of the C180 family. Similarly, another KIR2DS2*005 cell reported by Dr CK Hurley also lacked the same three genes (RDP76, Table 1). Furthermore, we found KIR2DS2*005 in one of two transplant patients in whom that gene combination was observed (LH236, Table 1). Therefore, the KIR phenotype 2DS2pos-(2DL2-2DL5B-2DS3)neg is a good marker of KIR2DS2*005-associated haplotypes; however, deletion of KIR2DL2, their most conspicuous trait, may be concealed in some cases by presence of this gene in the other chromosome (for example, C180, Figure 1). An identical gene combination has recently been reported to segregate in 1.2% of Northern Irish,12 who might carry KIR2DS2*005-associated haplotypes.
We assessed for possible existence of a hybrid gene mirroring KIR2DS2*005 (that is a hypothetical KIR gene combining a 5′ region similar to KIR2DS3 with a 3′ region similar to KIR2DS2). To that end, KIR2DS3-positive DNAs were studied with PCR–SSP primers targeting locus-specific sequences of intron 6 (an approach similar to that used here for KIR2DS2*005 typing). No DNA turned positive in the screening of 67 healthy controls and 75 transplant samples; these KIR2DS3-positive DNAs derived from 400 individuals, approximately, indicating that, if such hybrid gene exists, it is uncommon in Caucasoids. Negative results were also obtained in the study of 30 KIR2DS3-positive cells of different ethnicities from the DNA exchange organised by UCLA (http://www.hla.ucla.edu/cellDna.htm).
The KIR2DS2*005 product is expressed on the cell surface
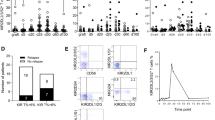
As KIR2DS2*005 shares part of its primary structure with KIR2DS3, and the latter KIR has been proposed to be retained intracellularly,10 it is of interest to determine the expression of KIR2DS2*005, which has never been investigated. KIR2DS2 products are difficult to identify in fresh uncloned NK cells because monoclonal antibodies (mAbs) monospecific for KIR2DS2 are lacking—all the available mAbs that detect KIR2DS2 also recognise its inhibitory homologues KIR2DL2 and KIR2DL3. A further complication is that most genomes having KIR2DS2 carry also KIR2DL2 and, often, KIR2DL3. To assess for surface expression of KIR2DS2*005, we used a flow cytometry approach similar to that reported by Pende et al.13 mAb 180701, specific for KIR2DL3, was combined with mAb DX27, which stains all three KIR2DS2, KIR2DL2 and KIR2DL3, to discriminate between the subpopulations that express KIR2DL3 and/or KIR2DL2/S2 (Figure 4).
KIR2DS2*005 is expressed on the cell surface. Expression of KIR2DS2, KIR2DL2 and KIR2DL3 was assessed by flow cytometry in NK cells and T-lymphocytes of individual C180F2 and three donors with different KIR2DS2/2DL2/2DL3 genotypes, using a combination of two monoclonal antibodies: DX27 (anti-KIR2DS2, -KIR2DL2 and -KIR2DL3) and 180701 (anti-KIR2DL3). The DX27+–180701− subpopulation represents cell expressing KIR2DS2/L2 in the absence of KIR2DL3. The DX27+–180701+ fraction represents cells expressing KIR2DL3; possible presence of KIR2DS2/L2 is concealed in this subpopulation, and it cannot be demonstrated by flow cytometry with the available antibodies.
Flow cytometry analysis of peripheral blood mononuclear cells (PBMCs) of donor C180F2, who has KIR2DS2*005 and KIR2DL3 but lacks KIR2DL2, and of control cells, defined up to three subpopulations: double negative cells, which lack expression of the three receptors; cells positive for both antibodies, which express KIR2DL3, with or without KIR2DL/S2 (concealed in these cells); and cells that bind DX27 but not 180701. In general, the last subpopulation contains cells that express KIR2DS2, KIR2DL2, or both, but not KIR2DL3. In donor C180F2, who lacks a KIR2DL2 gene, the DX27+–180701− subpopulation represents cells expressing only KIR2DS2, demonstrating surface expression of allele KIR2DS2*005 (Figure 4). Furthermore, the density of the receptor on the surface, as assessed by the median fluorescence intensity achieved with antibody DX27, is not dissimilar to those of the KIR2DL3 and KIR2DL2/S2 populations seen in the same and in other cells.
Discussion
KIRs evolve rapidly, driven by selective pressure of pathogens that tamper HLA expression.4 Ancestral KIR genes mutate through multiple mechanisms, which often obscure orthology relationships between the KIR of different species. Accumulation of successive amino-acid substitutions results in progressive change of the receptor function, including loss or modification of old properties and acquisition of new ones. Through this mechanism, KIR2DS2 appears to have lost an ancestral capacity to recognise MHC-C ligands with asparagine in position 80 (the ‘C1’ epitope), specificity still seen in its inhibitory homologues in humans (KIR2DL2 and, more distantly related, KIR2DL3) and in several activating and inhibitory KIR of apes.7
Besides point mutation, species acquire new KIR with novel functions more rapidly by asymmetric recombination. This leads, in first place, to haplotypes with gene loss and gene duplication,14, 15 which seem the origin of KIR2DS2 and KIR2DL2, encoded by paralogous genes. When recombination takes place within a gene, the resulting hybrids combine features (binding, signalling and mRNA expression) of the receptors that participated in the recombination. Through the latter mechanism, the KIR2DS2 ancestor is believed to have acquired the activating tail that characterises this receptor.16 Our results indicate that subsequent crossing over introduced the C-terminal region of KIR2DS3 into a KIR2DS2 gene, resulting in the chimerical allele KIR2DS2*005 (Figure 5). This origin is evident from the distribution of sequence homologies of this allele in intron 6 (Figure 2).
Evolutionary origin of KIR2DS2*005. Panel a represents a simplified pathway to explain the origin of KIR2DL2, KIR2DS2 and allele *005 of the latter gene. KIR2DS2*005 and its associated haplotype could derive from intrachromosomic recombination or from crossing over between chromatids (b, c). In the last case, two resulting recombinant haplotypes are predicted: one identical to that reported here; and one carrying three duplicated KIR genes, including a hypothetical hybrid gene made of the exons 1–6 of KIR2DS3 and exons 7–9 of KIR2DS2. We did not detect such gene in Spanish Caucasoids.
Another consequence of the asymmetric recombination was the loss of the genes that separate KIR2DS2 and KIR2DP1 in many KIR-B haplotypes—KIR2DL2, KIR2DL5B and KIR2DS3.6 We have found KIR2DS2*005 always associated with such KIR haplotype, possibly owing to recent origin of the allele. Also consistent with a recent origin is the fact that both the coding and the non-coding sequences of KIR2DS2*005 are still nearly identical to the homologous regions of other alleles of its ancestral genes (Figure 2). Two recombination mechanisms could have given rise to KIR2DS2*005. Intrachromosomic recombination would result in a KIR2DS2*005 haplotype with no reciprocal counterpart, since the excision circle would be lost (Figure 5b). In contrast, recombination between sister chromatids would yield two new haplotypes, each carrying a different chimerical gene and reciprocal sets of gene duplications or deletions (Figure 5c). We have proposed previously that such mechanism explains haplotypes carrying duplications or deletions of the same central genes in the KIR cluster—KIR3DP1, KIR2DL4 and KIR3DL1/S1.15 The counterpart of the KIR2DS2*005-associated haplotype would be the one carrying a chimera coding for the Ig-like domains of KIR2DS3 fused to a KIR2DS2 tail, and duplicated KIR2DL2, KIR2DL5B and KIR2DS3 genes. Such haplotypes are yet to be found, and we have failed to identify the putative chimerical KIR2DS3 allele using PCR primers for its predicted sixth intron. Of interest, another KIR2DS3 allele (*002) appears to have acquired exons 7–9 of KIR2DS5.11 KIR intron 6 thus seems prone to recombine, facilitating the exchange of the signalling and the ligand-binding regions between different KIR.
Prior to this study, the KIR2DS2*005 product had not been identified. Using flow cytometry with a combination of two mAbs of partially overlapping specificities (Figure 4), we have demonstrated KIR2DS2*005 expression on the surface of resting NK- and T-lymphocytes. Surface expression was previously uncertain because of sequence similarity with KIR2DS3, a receptor claimed to remain intracellular.10 Studies on the signalling capacity of the KIR2DS3 tail are lacking, but KIR2DS5,17 from which the intracellular tail of KIR2DS2*005 and KIR2DS3*001 differ by a single amino acid (asparagine for aspartate-271), triggers NK cytotoxicity and cytokine secretion upon antibody-mediated crosslink;18 therefore, KIR2DS2*005 is predictably an activating molecule.
The haplotype associated with KIR2DS2*005 gathers an unusual combination of features. First, it is one of the rare haplotypes that lack both KIR2DL2 and KIR2DL3, the genes encoding inhibitory receptors for the ‘HLA-C1’ epitope. Second, the KIR2DS2*005 function is unknown in two aspects: the KIR2DS2 ectodomain has lost virtually all binding capacity to HLA-C ligands, being unknown whether these have been substituted by altered or pathogen-encoded ligands.7 In KIR2DS2*005, that ectodomain is fused to a Cyt tail that diverges from those of canonical alleles and might transmit slightly different activating signals. This haplotype might therefore represent a step in an evolutionary process by which human NK cells replace an ancestral role, surveillance of MHC-C1 epitope expression through paired activating–inhibitory receptors, by a new one—detection of an unknown, potentially dangerous ligand by means of a novel activating receptor.
Materials and methods
Samples
Peripheral blood samples were collected from healthy donors after informed consent, and PBMCs were isolated by Ficoll-Hypaque density gradient centrifugation (Lymphoprep, Axis-Shield PoC AS, Oslo, Norway). DNA was purified using a salting-out method or a Maxwell 16 System (Promega, Madison, WI, USA). Total RNA was extracted from PBMCs using the RNeasy Plus Mini kit (Qiagen GmbH, D-40724, Hilden). Complementary DNA was synthesised from 1 μg of RNA using the Ready-to-Go T-Primed First Strand kit (Amersham Biosciences, Piscataway, NJ, USA).
Genetic analyses
Genotyping
Generic KIR typing, allelic KIR2DL5 and KIR2DS3 typing, and long-range PCR for initial KIR2DS2*005 identification were done as described.11, 19, 20 A new PCR–SSP method for specific detection of KIR2DS2*005 was designed: genomic DNA was amplified using 0.2 U of BioTaq DNA polymerase (Bioline, London, UK), and primers Fi6a+3019b (forward, 5′-TCTCGGGTAGAACAGCAA-3′) and Ri6a+3653b (reverse, 5′-CGAGGCTCAAGTTTTCCTTT-3′), which recognise a unique combination of polymorphisms in KIR2DS2*005 intron 6. Each reaction was supplemented with primers COCH (cochlin gene)-Fi11-80 (forward, 5′-CGCTGCAACTATGTAACAGT-3′) and COCH-Re12+64 (reverse, 5′-TCAGATTGGTCTTTCCACATGA-3′) to generate an internal positive control band of ∼1 kbp. PCR conditions were: initial denaturation for 2 min at 95 °C, then 10 cycles of 10 s at 94 °C and 40 s at 65 °C; and 20 cycles of 20 s at 94 °C, 20 s at 61 °C and 30 s at 72 °C. Using a similar approach, we searched possible existence of a putative reciprocal chimera containing a KIR2DS3 ectodomain fused to a KIR2DS2 tail; to this end, specific primers were replaced by Fi6c+2912 (forward, 5′-GAGATCAGCGTCAGCAC-3′) and Ri6c+3658 (reverse, 5′-GTTACCGAGGCTCAAGTG-3′) in the same PCR conditions.
Allelic typing of KIR3DL1 was performed by sequencing directly a 3.5 kbp amplicon generated with primers Fcon187 (5′-CGCTGTGGTGCCTCGA-3′) and Rc959 (5′-CMACTCGTAGGGAGAGTG-3′), using 0.2 U of LA-Taq (Hot Start version, Takara Bio Inc, Otsu, Shiga, Japan) in the following PCR conditions: 1 min at 94 °C, then 10 cycles of 10 s at 98 °C, 20 s at 66 °C and 4 min at 72 °C; and 20 cycles of 10 s at 98 °C, 20 s at 63 °C and 4 min at 72 °C. Nucleotide sequences of exons 3, 4 and 5 were determined with internal oligonucleotide primers different from those used in the PCR (details available upon request).
Genomic characterisation of KIR2DS2*005 and flanking genes
A 7.7 kbp-long genomic DNA segment, comprising exons 5–7 of KIR2DS2*005 and the intervening introns, was amplified with polymerase LA Taq (Takara Bio Inc) using PCR primers LFt970 (5′-GCTCTTTCCGTGACTCTCCCTAT-3′) and LRg1160 (5′-CTGCTTCGTGAGACTTACTTTTTTTGTC-3′), and analysed with internal sequencing primers. PCR conditions were 2 min at 95 °C, 10 cycles of 10 s at 94 °C, 10 min at 72 °C and 20 cycles of 10 s at 94 °C, 10 min at 70 °C.
The KIR genes flanking KIR2DS2*005 in donor C180F2 genomic DNA were determined using KIR-gene walking protocols similar to those we have reported previously11—the intergenic regions of interest, along with the preceding and consecutive exons of the flanking KIR genes, were amplified by combining one gene-specific and one KIR-generic primer in a long-range PCR in the following cycling conditions: 2 min at 95 °C, 10 cycles of 10 s at 95 °C, 10 min at 72 °C and 20 cycles of 10 s at 95 °C, 10 min at 68 °C. To identify the gene upstream of KIR2DS2, we used PCR primers LFcon1594 (forward, 5′-AATGTCTAAGGTCCCCACTGCC-3′) and LRa546 (reverse, 5′-CTCCAATGAGGTGCAAAGTGTCCTTAT-3′); for that located 3′ of it, primers LFg1160 (forward, 5′-CCTTCATCGCTGGTGCTCCG-3′) and LRcon649 (reverse, 5′-GGGWGTGAGKAACAGAACCGTAG-3′). Gene order was determined by sequence analysis of the exons encompassed in the PCR products.
Isolation of KIR2DS2*005 cDNA
The complete coding region of KIR2DS2*005 was amplified from the cDNA of donor C180F2 PBMCs, using 1.25 U of LA-Taq (Takara Bio Inc) and PCR primers recognising the 5′- and 3′-untranslated regions: KIR-generic forward primer Fcon28 (5′-GCGCTGCTGAGCTGAG-3′) and reverse primer Rg1340 (5′-ATTTGGAAGTTCTATGTACATGCTGC-3′), specific for KIR2DS2*005 and KIR2DS3*001. PCR conditions were: 5 min at 96 °C, then 10 cycles of 10 s at 94 °C, 25 s at 69 °C and 2 min at 72 °C; and 35 cycles of 10 s at 94 °C, 25 s at 65 °C and 2 min at 72 °C. A 1023 bp-long amplicon was obtained and sequenced with internal primers.
DNA sequencing
All PCR products were submitted to direct nucleotide sequencing with no cloning step. Previously, residual dNTP and PCR primers were eliminated with ExoSAP-IT (USB Corporation, Cleveland, OH, USA) following the provider guidelines. PCR products were then sequenced using BigDye terminator v3.1 or v1.1 cycle sequencing kits (Applied Biosystems, Foster City, CA, USA). Reactions were processed in a 3100-Avant automated DNA sequencer (Applied Biosystems) in the central DNA sequencing facility of Hospital Universitario Puerta de Hierro. Nucleotide sequences of KIR2DS2*005 were deposited in the EMBL database under the accession numbers FR750993–FR750995.
Antibodies and flow cytometry
PBMCs of donors with known KIR genotypes were incubated with a combination of the following mAbs: fluorescein-isothiocyanate-labelled anti-human KIR2DL3 (180701, mouse IgG2a, R&D Systems, Minneapolis, MN, USA), phyco-erythrin-labelled anti-human KIR2DS2/L2/L3 (DX27, mouse IgG2a, Miltenyi Biotech, Bergisch Gladbach, Germany); and either tri-colour-labelled anti-human CD3 (S4.1, mouse IgG2a, Invitrogen, Camarillo, CA, USA) or tri-colour-labelled anti-human CD16 (3G8, mouse IgG1, Invitrogen) to identify T-lymphocytes and the CD16+ fraction of NK cells, respectively. Isotype-matched negative controls were: fluorescein-isothiocyanate-labelled IgG2a (Invitrogen), phyco-erythrin-labelled IgG2a (S43.10, Miltenyi Biotech) and tri-colour-labelled IgG1 (Invitrogen). Flow cytometry analysis was made in an Epics XL apparatus and represented with the Expo-32 software (Beckman Coulter, Brea, CA, USA).
References
Moretta L, Moretta A . Killer immunoglobulin-like receptors. Curr Opin Immunol 2004; 16: 626–633.
Wilson MJ, Torkar M, Haude A, Milne S, Jones T, Sheer D et al. Plasticity in the organization and sequences of human KIR/ILT gene families. Proc Natl Acad Sci USA 2000; 97: 4778–4783.
Marsh SG, Parham P, Dupont B, Geraghty DE, Trowsdale J, Middleton D et al. Killer-cell immunoglobulin-like receptor (KIR) nomenclature report, 2002. Tissue Antigens 2003; 62: 79–86.
Vilches C, Parham P . KIR: diverse, rapidly evolving receptors of innate and adaptive immunity. Annu Rev Immunol 2002; 20: 217–251.
Abi-Rached L, Moesta AK, Rajalingam R, Guethlein LA, Parham P . Human-specific evolution and adaptation led to major qualitative differences in the variable receptors of human and chimpanzee natural killer cells. PLoS Genet 2010; 6: e1001192.
Pyo CW, Guethlein LA, Vu Q, Wang R, Abi-Rached L, Norman PJ et al. Different patterns of evolution in the centromeric and telomeric regions of group A and B haplotypes of the human killer cell Ig-like receptor locus. PLoS One 2010; 5: e15115.
Moesta AK, Graef T, Abi-Rached L, Older Aguilar AM, Guethlein LA, Parham P . Humans differ from other hominids in lacking an activating NK cell receptor that recognizes the C1 epitope of MHC class I. J Immunol 2010; 185: 4233–4237.
Robinson J, Mistry K, McWilliam H, Lopez R, Marsh SG . IPD—the Immuno Polymorphism Database. Nucleic Acids Res 2009; 38: D863–D869.
Rajalingam R, Gardiner CM, Canavez F, Vilches C, Parham P . Identification of seventeen novel KIR variants: fourteen of them from two non-Caucasian donors. Tissue Antigens 2001; 57: 22–31.
VandenBussche CJ, Mulrooney TJ, Frazier WR, Dakshanamurthy S, Hurley CK . Dramatically reduced surface expression of NK cell receptor KIR2DS3 is attributed to multiple residues throughout the molecule. Genes Immun 2009; 10: 162–173.
Ordóñez D, Meenagh A, Gómez-Lozano N, Castaño J, Middleton D, Vilches C . Duplication, mutation and recombination of the human orphan gene KIR2DS3 contribute to the diversity of KIR haplotypes. Genes Immun 2008; 9: 431–437.
Gourraud PA, Meenagh A, Cambon-Thomsen A, Middleton D . Linkage disequilibrium organization of the human KIR superlocus: implications for KIR data analyses. Immunogenetics 2010; 62: 729–740.
Pende D, Marcenaro S, Falco M, Martini S, Bernardo ME, Montagna D et al. Anti-leukemia activity of alloreactive NK cells in KIR ligand-mismatched haploidentical HSCT for pediatric patients: evaluation of the functional role of activating KIR and redefinition of inhibitory KIR specificity. Blood 2009; 113: 3119–3129.
Gómez-Lozano N, de Pablo R, Puente S, Vilches C . Recognition of HLA-G by the NK cell receptor KIR2DL4 is not essential for human reproduction. Eur J Immunol 2003; 33: 639–644.
Gómez-Lozano N, Estefanía E, Williams F, Halfpenny I, Middleton D, Solís R et al. The silent KIR3DP1 gene (CD158c) is transcribed and might encode a secreted receptor in a minority of humans, in whom the KIR3DP1, KIR2DL4 and KIR3DL1/KIR3DS1 genes are duplicated. Eur J Immunol 2005; 35: 16–24.
Abi-Rached L, Parham P . Natural selection drives recurrent formation of activating killer cell immunoglobulin-like receptor and Ly49 from inhibitory homologues. J Exp Med 2005; 201: 1319–1332.
Vilches C, Pando MJ, Rajalingam R, Gardiner CM, Parham P . Discovery of two novel variants of KIR2DS5 reveals this gene to be a common component of human KIR ‘B’ haplotypes. Tissue Antigens 2000; 56: 453–456.
Della Chiesa M, Romeo E, Falco M, Balsamo M, Augugliaro R, Moretta L et al. Evidence that the KIR2DS5 gene codes for a surface receptor triggering natural killer cell function. Eur J Immunol 2008; 38: 2284–2289.
Vilches C, Castaño J, Gómez-Lozano N, Estefanía E . Facilitation of KIR genotyping by a PCR-SSP method that amplifies short DNA fragments. Tissue Antigens 2007; 70: 415–422.
Gómez-Lozano N, Trompeter HI, de Pablo R, Estefanía E, Uhrberg M, Vilches C . Epigenetic silencing of potentially functional KIR2DL5 alleles: implications for the acquisition of KIR repertoires by NK cells. Eur J Immunol 2007; 37: 1954–1965.
Acknowledgements
This work was supported by grants BFU2005–04622 and SAF2010-22153-C03-03, from the Spanish Ministerio de Ciencia e Innovación. We thank María Cañizares for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Ordóñez, D., Gómez-Lozano, N., Rosales, L. et al. Molecular characterisation of KIR2DS2*005, a fusion gene associated with a shortened KIR haplotype. Genes Immun 12, 544–551 (2011). https://doi.org/10.1038/gene.2011.35
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/gene.2011.35
Keywords
This article is cited by
-
Comparative genetics of KIR haplotype diversity in humans and rhesus macaques: the balancing act
Immunogenetics (2022)
-
Revealing complete complex KIR haplotypes phased by long-read sequencing technology
Genes & Immunity (2017)
-
Recombinant structures expand and contract inter and intragenic diversification at the KIR locus
BMC Genomics (2013)