Abstract
Glandular neoplasms involving the urinary bladder carry a challenging differential diagnosis including primary and secondary processes. We investigated the potential diagnostic utility of cadherin-17 and GATA3 in 25 primary adenocarcinomas of the urinary bladder, as compared with other commonly used markers including β-catenin and p63. Urothelial carcinoma with glandular differentiation (11), colorectal adenocarcinoma secondarily involving the bladder (25), and primary colorectal adenocarcinoma (22) were also analyzed and the results were compared using a Fisher exact test. Cadherin-17 was expressed in 23/25 primary bladder adenocarcinomas (92%), 23/25 colorectal adenocarcinomas involving the bladder (92%), 21/22 primary colorectal adenocarcinomas (95%) and entirely negative (0/11) in both components of urothelial carcinoma with glandular differentiation (P<0.001). In urothelial carcinoma with glandular differentiation, positive nuclear staining for GATA3 was evident in the urothelial component for 18% (2/11) and the glandular component for 9% (1/11) with additional tumors showing only cytoplasmic staining. Nuclear reactivity for GATA3 was not present in primary bladder adenocarcinoma and primary/secondary colorectal adenocarcinoma (P<0.05). Positive nuclear and cytoplasmic immunostaining for β-catenin was evident in 21/22 primary colorectal adenocarcinomas (95%) and 23/25 cases of secondary involvement by colorectal adenocarcinoma (92%). In contrast, positive membranous and cytoplasmic staining for β-catenin was observed in 23/25 primary bladder adenocarcinomas (92%) and 11/11 urothelial carcinomas with glandular differentiation (100%, P<0.001). p63 was expressed only in the urothelial component of urothelial carcinoma with glandular differentiation and not in the glandular component (P<0.001). In summary, cadherin-17 is a relatively specific and sensitive marker for primary adenocarcinoma of the urinary bladder, distinguishing it from urothelial carcinoma with glandular differentiation. However, it does not distinguish primary bladder adenocarcinoma from secondary involvement by colorectal adenocarcinoma. The pattern of reactivity for β-catenin remains the most useful marker for distinguishing these two tumors.
Similar content being viewed by others
Main
Primary adenocarcinoma of the urinary bladder is an uncommon neoplasm, accounting for 0.5–2.0% of all malignant bladder tumors.1, 2, 3 Morphologically, these tumors show a wide spectrum of histologic appearances, including colonic (enteric), mucinous (so-called ‘colloid’), signet ring cell, clear cell, mixed, and not otherwise specified subtypes.1, 4, 5, 6 Therefore, distinguishing them from the secondary involvement of the urinary bladder by adenocarcinoma of another organ, especially the colon, is often challenging or impossible by light microscopy alone.6
Although immunohistochemical markers such as CK7, CK20, villin, CDX-2, and β-catenin have been proposed to aid in resolving the differential diagnosis of these tumors, limitations in sensitivity and specificity have prevented any single marker or small panel of markers from emerging as clearly superior.6, 7, 8, 9, 10 Differential diagnostic challenges remain and the identification of additional diagnostic tools is necessary. Cadherin-17 (CDH17), also called liver–intestinal cadherin or human peptide transporter-1, has been recently recognized as a sensitive and specific marker for adenocarcinoma of the digestive system, especially colorectal adenocarcinoma.11, 12, 13, 14, 15 Conversely, GATA3, a transcription factor, is a newly described marker of urothelial carcinoma.16, 17, 18 However, the expression of cadherin-17 and GATA3 in primary adenocarcinoma of the urinary bladder has not been well characterized. The aim of this study was to investigate the expression of cadherin-17 and GATA3 in primary adenocarcinoma of the urinary bladder and evaluate the potential diagnostic usefulness of these markers along with other commonly used markers such as β-catenin and p63.
Materials and methods
Cases
A total of 25 primary urinary bladder adenocarcinomas were analyzed including 19 colonic (enteric), three signet ring cell, and three mixed types. For comparison, 11 urothelial carcinomas with prominent glandular differentiation, 25 colorectal adenocarcinomas secondarily involving the urinary bladder, and 22 primary colorectal adenocarcinomas were analyzed. Clinical histories and hematoxylin and eosin (H&E) slides of each case were reviewed to confirm the tumor origin.
Immunohistochemistry
Immunostaining was performed using the Dako Autostainer Plus (Dako, Carpinteria, CA, USA). The following antibodies were used: cadherin-17 (Novus, Littleton, CO, USA; 1:500 dilution), GATA3 (BioCare Medical, Concord, CA, USA; 1:50 dilution), p63 (Dako; 1:400 dilution), and β-catenin (Cell Marque, Rockland, CA, USA; prediluted/ready to use). Immunoreaction was performed using the EnVision Flex and Flex+Visualization Systems (Dako). Diaminobenzidine (3,3-diaminobenzidine) was used as the chromogen. Positive and negative controls stained appropriately.
The interpretation of immunoreactivity was performed in a semiquantitative manner by analyzing the extent of the positively staining tumor cells. The interpretation was performed as follows: 0 or negative, 0% tumor cell positivity; 1+ or focal, 1–10% tumor cell positivity; 2+ or moderate, 11–50% tumor cell positivity; and 3+ or diffuse, >50% tumor cell positivity. Results of immunohistochemical staining were analyzed with a two-tailed Fisher exact test. A P value of <0.05 was considered statistically significant.
Results
Results of immunohistochemical staining are summarized in Table 1.
Nonneoplastic Tissue Adjacent to the Tumor
In nonneoplastic tissues, strong, diffuse membranous staining for cadherin-17 was detected exclusively in colorectal mucosa. GATA3 and p63 were exclusively expressed in nuclei of normal urothelium. Positive membranous staining for β-catenin was seen in both colorectal mucosa and urothelium.
Primary Adenocarcinoma of the Urinary Bladder
Cadherin-17 and β-catenin were each expressed in 92% (23/25) of tumors (Figure 1). The staining pattern was membranous for cadherin-17 and membranous and cytoplasmic for β-catenin, (Figures 1 and 2) although two tumors showed nuclear and cytoplasmic staining with antibody to β-catenin. There was a statistically significant difference in staining pattern (membranous and cytoplasmic vs nuclear) when compared with colorectal adenocarcinoma (P<0.001). A variable percentage of tumor cells showed a positive reaction (cadherin-17: mean, 62%; β-catenin: mean, 90%). Staining for p63 was negative in all tumors. Unlike the nuclear expression of GATA3 in normal urothelium, primary urinary bladder adenocarcinoma showed only cytoplasmic reactivity, present in 60% of tumors (15/25). Differences in immunoreactivity between different subtypes of primary bladder adenocarcinoma were not observed.
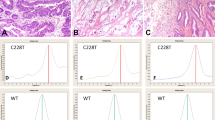
Expression of cadherin-17 (CDH17) and β-catenin (β-cat) in primary adenocarcinoma of the urinary bladder and colorectal adenocarcinoma involving the bladder: (a) Hematoxylin and eosin-stained section of primary adenocarcinoma of the urinary bladder (colonic type) corresponding to the immunohistochemistry, which showed strong membranous staining with cadherin-17 (b) and membranous and cytoplasmic staining for β-catenin (c). Primary adenocarcinoma of the urinary bladder, signet ring cell type (d), also showed strong membranous staining with cadherin-17 (e) and membranous and cytoplasmic staining for β-catenin (f). In contrast, colorectal adenocarcinoma involving the bladder (g) showed strong membranous staining with cadherin-17 (h) paired with nuclear and cytoplasmic staining for β-catenin (i). Colorectal adenocarcinoma involving the bladder, signet ring cell type (J), also showed membranous staining with cadherin-17 (k) and nuclear and cytoplasmic staining for β-catenin (l).
Expression patterns of β-catenin (β-cat) in primary adenocarcinoma of the urinary bladder and colorectal adenocarcinoma involving the bladder: (a) Hematoxylin and eosin-stained section of primary adenocarcinoma of the urinary bladder (colonic type) corresponding to the immunohistochemistry. The tumor showed membranous and cytoplasmic staining with β-catenin (b). In contrast, colorectal adenocarcinoma involving the bladder (c) showed nuclear and cytoplasmic staining for β-catenin (d).
Urothelial Carcinoma with Glandular Differentiation
None of the 11 cases of urothelial carcinoma with glandular differentiation was positive for cadherin-17 in either the urothelial or glandular component, yielding no significant difference when compared with each other but a statistically significant difference when compared with adenocarcinomas (colorectal and primary urinary bladder adenocarcinomas, P<0.001). β-catenin showed membranous and cytoplasmic reactivity in both urothelial and glandular components of all 11 tumors (Figure 3). Therefore, no statistically significant difference was detected when comparing the two components to each other; however, a statistically significant difference was detected when compared with the nuclear staining pattern in colorectal adenocarcinomas (P<0.001). Positive staining for p63 was present in the urothelial component for 73% (8/11; nuclear staining), but negative in the glandular component of all cases (P<0.001). Positive nuclear staining for GATA3 was evident in the urothelial component for 2/11 tumors (18%) and only a single tumor in the glandular component (9%). No statistically significant difference was detected between the urothelial and glandular components; however, urothelial carcinomas showed increased staining when compared with pure adenocarcinomas (Table 1). Cytoplasmic staining for GATA3 was also present in some cases (36%).
Expression of β-catenin (β-cat) and GATA3 in urothelial carcinomas with prominent glandular differentiation: (a) Hematoxylin and eosin-stained section of the urothelial component of urothelial carcinoma with glandular differentiation. The urothelial carcinoma component showed membranous and cytoplasmic staining with β-catenin (b) and nuclear staining for GATA3 (c). The glandular component of this tumor (d) showed membranous and cytoplasmic staining with β-catenin (e) and nuclear staining for GATA3 (F), although nuclear expression of GATA3 was lacking in the majority of cases of urothelial carcinoma with glandular differentiation.
Secondary Involvement by Colorectal Adenocarcinoma
Cadherin-17 and β-catenin were each expressed in 92% of tumors (23/25) (Figures 1 and 2), resulting in no statistically significant difference when compared with primary urinary bladder adenocarcinoma (P=1). Cadherin-17 labeled the tumor cells in a membranous pattern, while β-catenin showed nuclear and cytoplasmic staining. Some cases also showed concurrent membranous staining for β-catenin. Membranous reactivity without nuclear labeling for β-catenin was present in two tumors. A variable percentage of cells showed a positive reaction (cadherin-17: mean, 62%; β-catenin: mean, 78%). All cases were negative for p63 and nuclear expression of GATA3 was not present in any tumor, although cytoplasmic positivity for GATA3 was present in some tumors (48%).
Primary Colorectal Adenocarcinoma
Membranous staining for cadherin-17 was observed in 95% (21/22) of primary colorectal adenocarcinomas with a mean of 80% of tumor cells showing a positive reaction. None showed nuclear staining for GATA3 or p63, yielding no significant difference in p63 staining when compared with secondary adenocarcinoma or primary urinary bladder adenocarcinoma. Cytoplasmic labeling with GATA3 was frequently present (86%). β-Catenin was expressed in the nuclei and cytoplasm of 21 primary colorectal adenocarcinomas with a mean of 97% of tumor cells showing a positive reaction. A single case showed only membranous and cytoplasmic staining for β-catenin.
Discussion
In this study, we have extended the known immunohistochemical profile of primary adenocarcinoma of the urinary bladder and confirmed some prior observations regarding these neoplasms.3, 7, 19, 20 Cadherin-17, also called liver–intestinal cadherin or human peptide transporter-1, is a sensitive and specific marker for adenocarcinomas of the digestive system, especially colorectal adenocarcinomas.11, 12, 13, 14, 15 In the study of Su et al,12 expression of cadherin-17 was very common in colorectal adenocarcinomas (96%) and evident in more than half of gastric and pancreatic adenocarcinomas. In contrast, cadherin-17 expression was very uncommon in carcinomas arising outside the gastrointestinal tract. Of 331 tumors including 44 breast cancers, 39 uterine cervical carcinomas, 50 lung cancers, 47 renal cell carcinomas, 89 ovarian cancers, and 39 prostatic cancers; cadherin-17 was expressed in only one (0.3%) prostatic adenocarcinoma.12 These results support the utility of cadherin-17 as a useful diagnostic marker of gastrointestinal adenocarcinoma. Park et al21 similarly found a high concordance between the staining reactions in primary and metastatic colorectal carcinomas. In this study, we report that in addition to both primary and secondary colorectal adenocarcinoma, cadherin-17 is also commonly expressed in primary adenocarcinoma of the urinary bladder (92%) and negative in urothelial carcinoma with glandular differentiation, a statistically significant difference (P<0.001). Based upon these findings, cadherin-17 may be useful as a relatively specific and sensitive marker in the diagnosis of primary adenocarcinoma of the urinary bladder, distinguishing it from urothelial carcinoma with glandular differentiation. However, this marker does not distinguish primary adenocarcinoma of the urinary bladder from secondary involvement by colorectal adenocarcinoma.
Immunohistochemical antibody to transcription factor GATA3 has recently been recognized as a useful marker of urothelial differentiation.16, 17, 18 Unlike the usual nuclear expression pattern of GATA3 in normal urothelium and urothelial carcinoma, we identified only cytoplasmic reactivity in primary adenocarcinoma of the urinary bladder (60%), colorectal adenocarcinoma (86%), and secondary involvement of the urinary bladder by colorectal adenocarcinoma (48%); nuclear expression was lacking. Previous studies have suggested a close histogenetic and pathogenetic relationship of primary adenocarcinoma of the urinary bladder and colorectal adenocarcinoma.22, 23, 24, 25, 26 Our finding that cadherin-17 and GATA3 expression are very similar in these two tumor types supports this relationship (Table 2). However, positive nuclear and cytoplasmic immunostaining for β-catenin was evident in 95% of (21/22) primary and 92% of (23/25) secondary colorectal adenocarcinoma, in contrast to positive membranous and cytoplasmic staining for β-catenin in 92% of (23/25) primary adenocarcinomas of the urinary bladder and 100% of (11/11) urothelial carcinomas with glandular differentiation (P<0.001). These data indicate that dysregulation of β-catenin, an important aberration seen in colorectal carcinogenesis, does not appear to play a role in the pathogenesis of the bladder adenocarcinoma,27, 28 confirming previous observations regarding the immunohistochemical profile of primary adenocarcinoma of the urinary bladder.3, 7, 19, 20 β-Catenin remains the most sensitive and specific marker for distinguishing primary adenocarcinoma of the urinary bladder from secondary involvement by colorectal adenocarcinoma.
We also evaluated this panel of biomarkers in 11 urothelial carcinomas with glandular differentiation. Of interest, p63 was expressed only in the urothelial carcinoma component (8/11 tumors) and not in the glandular component (P<0.001). GATA3 showed expression only in a minority of cases (two in the urothelial component and one in the glandular component), a smaller percentage of tumors than for urothelial carcinoma in general, which has been reported to be as high as 67–86%.16, 18 Cadherin-17 was not expressed in either component of any tumor, contrasting to almost all primary adenocarcinomas of the urinary bladder, which were positive for cadherin-17 (P<0.001). These prominent immunophenotypic differences between urothelial carcinoma with glandular differentiation and primary adenocarcinoma suggest that distinct mechanisms of histogenesis and pathogenesis are at play in the development of these tumors, despite histologic overlap in features.
In summary, in this study we have extended the immunohistochemical profile of primary adenocarcinoma of the urinary bladder (cadherin-17 positive, GATA3 negative, β-catenin membranous and cytoplasmic positive). Cadherin-17 is a relatively specific and sensitive marker in diagnosing primary adenocarcinoma of the urinary bladder, distinguishing it from urothelial carcinoma with glandular differentiation. However, it does not distinguish primary adenocarcinoma of the urinary bladder from secondary involvement by colorectal adenocarcinoma, in which case, β-catenin appears to remain the most useful marker. Although GATA3, when positive, supports a diagnosis of urothelial carcinoma with glandular differentiation, nuclear expression is only present in a minority of cases.
References
Cheng L, Lopez-Beltran A, Bostwick DG . Bladder Pathology. Wiley-Blackwell: Hoboken, NJ, 2012.
Grignon DJ, Ro JY, Ayala AG et al Primary adenocarcinoma of the urinary bladder. A clinicopathologic analysis of 72 cases. Cancer 1991;67:2165–2172.
Thomas AA, Stephenson AJ, Campbell SC et al Clinicopathologic features and utility of immunohistochemical markers in signet-ring cell adenocarcinoma of the bladder. Hum Pathol 2009;40:108–116.
Chor PJ, Gaum LD, Young RH . Clear cell adenocarcinoma of the urinary bladder: report of a case of probable mullerian origin. Mod Pathol 1993;6:225–228.
Roy S, Parwani AV . Adenocarcinoma of the urinary bladder. Arch Pathol Lab Med 2011;135:1601–1605.
Williamson SR, Lopez-Beltran A, Montironi R et al Glandular lesions of the urinary bladder: clinical significance and differential diagnosis. Histopathology 2011;58:811–834.
Wang HL, Lu DW, Yerian LM et al Immunohistochemical distinction between primary adenocarcinoma of the bladder and secondary colorectal adenocarcinoma. Am J Surg Pathol 2001;25:1380–1387.
Tamboli P, Mohsin SK, Hailemariam S et al Colonic adenocarcinoma metastatic to the urinary tract versus primary tumors of the urinary tract with glandular differentiation: a report of 7 cases and investigation using a limited immunohistochemical panel. Arch Pathol Lab Med 2002;126:1057–1063.
Suh N, Yang XJ, Tretiakova MS et al Value of CDX2, villin, and alpha-methylacyl coenzyme A racemase immunostains in the distinction between primary adenocarcinoma of the bladder and secondary colorectal adenocarcinoma. Mod Pathol 2005;18:1217–1222.
Raspollini MR, Nesi G, Baroni G et al Immunohistochemistry in the differential diagnosis between primary and secondary intestinal adenocarcinoma of the urinary bladder. Appl Immunohistochem Mol Morphol 2005;13:358–362.
Ito R, Oue N, Yoshida K et al Clinicopathological significant and prognostic influence of cadherin-17 expression in gastric cancer. Virchows Arch 2005;447:717–722.
Su MC, Yuan RH, Lin CY et al Cadherin-17 is a useful diagnostic marker for adenocarcinomas of the digestive system. Mod Pathol 2008;21:1379–1386.
Panarelli NC, Yantiss RK, Yeh MM et al Tissue-specific cadherin CDH17 is a useful marker of gastrointestinal adenocarcinomas with higher sensitivity than CDX2. Am J Clin Pathol 2012;138:211–222.
Ge J, Chen Z, Wu S et al A clinicopathological study on the expression of cadherin-17 and caudal-related homeobox transcription factor (CDX2) in human gastric carcinoma. Clin Oncol (R Coll Radiol) 2008;20:275–283.
Lee HJ, Nam KT, Park HS et al Gene expression profiling of metaplastic lineages identifies CDH17 as a prognostic marker in early stage gastric cancer. Gastroenterology 2010;139:213–25 e3.
Higgins JP, Kaygusuz G, Wang L et al Placental S100 (S100P) and GATA3: markers for transitional epithelium and urothelial carcinoma discovered by complementary DNA microarray. Am J Surg Pathol 2007;31:673–680.
Esheba GE, Longacre TA, Atkins KA et al Expression of the urothelial differentiation markers GATA3 and placental S100 (S100P) in female genital tract transitional cell proliferations. Am J Surg Pathol 2009;33:347–353.
Liu H, Shi J, Wilkerson ML et al Immunohistochemical evaluation of GATA3 expression in tumors and normal tissues: a useful immunomarker for breast and urothelial carcinomas. Am J Clin Pathol 2012;138:57–64.
Gopalan A, Sharp DS, Fine SW et al Urachal carcinoma: a clinicopathologic analysis of 24 cases with outcome correlation. Am J Surg Pathol 2009;33:659–668.
Del Sordo R, Bellezza G, Colella R et al Primary signet-ring cell carcinoma of the urinary bladder: a clinicopathologic and immunohistochemical study of 5 cases. Appl Immunohistochem Mol Morphol 2009;17:18–22.
Park JH, Seol JA, Choi HJ et al Comparison of cadherin-17 expression between primary colorectal adenocarcinomas and their corresponding metastases: the possibility of a diagnostic marker for detecting the primary site of metastatic tumour. Histopathology 2011;58:315–318.
Bullock PS, Thoni DE, Murphy WM . The significance of colonic mucosa (intestinal metaplasia) involving the urinary tract. Cancer 1987;59:2086–2090.
Bates AW, Baithun SI . Secondary neoplasms of the bladder are histological mimics of nontransitional cell primary tumours: clinicopathological and histological features of 282 cases. Histopathology 2000;36:32–40.
Nakanishi K, Tominaga S, Kawai T et al Mucin histochemistry in primary adenocarcinoma of the urinary bladder (of urachal or vesicular origin) and metastatic adenocarcinoma originating in the colorectum. Pathol Int 2000;50:297–303.
Sung MT, Lopez-Beltran A, Eble JN et al Divergent pathway of intestinal metaplasia and cystitis glandularis of the urinary bladder. Mod Pathol 2006;19:1395–1401.
Morton MJ, Zhang S, Lopez-Beltran A et al Telomere shortening and chromosomal abnormalities in intestinal metaplasia of the urinary bladder. Clin Cancer Res 2007;13:6232–6236.
Chung DC . The genetic basis of colorectal cancer: insights into critical pathways of tumorigenesis. Gastroenterology 2000;119:854–865.
Fearon ER . Human cancer syndromes: clues to the origin and nature of cancer. Science 1997;278:1043–1050.
Acknowledgements
This study was supported by National Natural Science Foundation of China (81101933; Q Rao); Natural Science Foundation of Jiangsu Province, China (BK2010463; Q Rao) and Jiangsu Government Scholarship for Overseas Studies (W Huang). We thank Tracey Bender for her excellent editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Rao, Q., Williamson, S., Lopez-Beltran, A. et al. Distinguishing primary adenocarcinoma of the urinary bladder from secondary involvement by colorectal adenocarcinoma: extended immunohistochemical profiles emphasizing novel markers. Mod Pathol 26, 725–732 (2013). https://doi.org/10.1038/modpathol.2012.229
Received:
Revised:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/modpathol.2012.229
Keywords
This article is cited by
-
Diagnostic Relevance of GATA 3 Expression in Urinary Bladder Carcinoma of Divergent Differentiation and Other Histological Variants
Indian Journal of Surgical Oncology (2021)
-
Next-generation sequencing-based molecular characterization of primary urinary bladder adenocarcinoma
Modern Pathology (2017)