Abstract
The innate immune system responds to infection and tissue damage by activating cytosolic sensory complexes called 'inflammasomes'. Cytosolic DNA is sensed by AIM2-like receptors (ALRs) during bacterial and viral infections and in autoimmune diseases. Subsequently, recruitment of the inflammasome adaptor ASC links ALRs to the activation of caspase-1. A controlled immune response is crucial for maintaining homeostasis, but the regulation of ALR inflammasomes is poorly understood. Here we identified the PYRIN domain (PYD)-only protein POP3, which competes with ASC for recruitment to ALRs, as an inhibitor of DNA virus–induced activation of ALR inflammasomes in vivo. Data obtained with a mouse model with macrophage-specific POP3 expression emphasize the importance of the regulation of ALR inflammasomes in monocytes and macrophages.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Accession codes
References
Martinon, F., Burns, K. & Tschopp, J. The Inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-1β. Mol. Cell 10, 417–426 (2002).
Stehlik, C. et al. Apoptosis-associated speck-like protein containing a caspase recruitment domain is a regulator of procaspase-1 activation. J. Immunol. 171, 6154–6163 (2003).
Srinivasula, S.M. et al. The PYRIN-CARD protein ASC is an activating adaptor for Caspase-1. J. Biol. Chem. 277, 21119–21122 (2002).
Barbalat, R., Ewald, S.E., Mouchess, M.L. & Barton, G.M. Nucleic acid recognition by the innate immune system. Annu. Rev. Immunol. 29, 185–214 (2011).
Fernandes-Alnemri, T. et al. The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nat. Immunol. 11, 385–393 (2010).
Rathinam, V.A. et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat. Immunol. 11, 395–402 (2010).
Jones, J.W. et al. Absent in melanoma 2 is required for innate immune recognition of Francisella tularensis. Proc. Natl. Acad. Sci. USA 107, 9771–9776 (2010).
Kerur, N. et al. IFI16 acts as a nuclear pathogen sensor to induce the inflammasome in response to kaposi sarcoma-associated herpesvirus infection. Cell Host Microbe 9, 363–375 (2011).
Albrecht, M., Choubey, D. & Lengauer, T. The HIN domain of IFI-200 proteins consists of two OB folds. Biochem. Biophys. Res. Commun. 327, 679–687 (2005).
Jin, T. et al. Structures of the HIN domain:DNA complexes reveal ligand binding and activation mechanisms of the AIM2 inflammasome and IFI16 receptor. Immunity 36, 561–571 (2012).
Bürckstümmer, T. et al. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat. Immunol. 10, 266–272 (2009).
Fernandes-Alnemri, T., Yu, J.W., Datta, P., Wu, J. & Alnemri, E.S. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature 458, 509–513 (2009).
Hornung, V. et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 458, 514–518 (2009).
Roberts, T.L. et al. HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science 323, 1057–1060 (2009).
Schattgen, S.A. & Fitzgerald, K.A. The PYHIN protein family as mediators of host defenses. Immunol. Rev. 243, 109–118 (2011).
Ludlow, L.E., Johnstone, R.W. & Clarke, C.J. The HIN-200 family: more than interferon-inducible genes? Exp. Cell Res. 308, 1–17 (2005).
Dorfleutner, A. et al. A Shope fibroma virus PYRIN-only protein modulates the host immune response. Virus Genes 35, 685–694 (2007).
Dorfleutner, A. et al. Cellular pyrin domain-only protein 2 is a candidate regulator of inflammasome activation. Infect. Immun. 75, 1484–1492 (2007).
Stehlik, C. et al. The PAAD/PYRIN-only protein POP1/ASC2 is a modulator of ASC-mediated NF-κB and pro-caspase-1 regulation. Biochem. J. 373, 101–113 (2003).
Stehlik, C. & Dorfleutner, A. COPs and POPs: modulators of inflammasome activity. J. Immunol. 179, 7993–7998 (2007).
Bedoya, F., Sandler, L.L. & Harton, J.A. Pyrin-only protein 2 modulates NF-κB and disrupts ASC:CLR interactions. J. Immunol. 178, 3837–3845 (2007).
Johnston, J.B. et al. A poxvirus-encoded pyrin domain protein interacts with ASC-1 to inhibit host inflammatory and apoptotic responses to infection. Immunity 23, 587–598 (2005).
Hiller, S. et al. NMR Structure of the apoptosis- and inflammation-related NALP1 pyrin domain. Structure 11, 1199–1205 (2003).
Jin, T., Perry, A., Smith, P., Jiang, J. & Xiao, T.S. Structure of the absent in melanoma 2 (AIM2) pyrin domain provides insights into the mechanisms of AIM2 autoinhibition and inflammasome assembly. J. Biol. Chem. 288, 13225–13235 (2013).
Boyden, E.D. & Dietrich, W.F. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat. Genet. 38, 240–244 (2006).
Faustin, B. et al. Reconstituted NALP1 inflammasome reveals two-step mechanism of caspase-1 activation. Mol. Cell 25, 713–724 (2007).
Martinon, F., Petrilli, V., Mayor, A., Tardivel, A. & Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440, 237–241 (2006).
Cassel, S.L. et al. The Nalp3 inflammasome is essential for the development of silicosis. Proc. Natl. Acad. Sci. USA 105, 9035–9040 (2008).
Dostert, C. et al. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 320, 674–677 (2008).
Hornung, V. et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 9, 847–856 (2008).
Franchi, L. et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1β in salmonella-infected macrophages. Nat. Immunol. 7, 576–582 (2006).
Miao, E.A. et al. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1β via Ipaf. Nat. Immunol. 7, 569–575 (2006).
Unterholzner, L. et al. IFI16 is an innate immune sensor for intracellular DNA. Nat. Immunol. 11, 997–1004 (2010).
Cridland, J.A. et al. The mammalian PYHIN gene family: phylogeny, evolution and expression. BMC Evol. Biol. 12, 140 (2012).
Gough, P.J., Gordon, S. & Greaves, D.R. The use of human CD68 transcriptional regulatory sequences to direct high-level expression of class A scavenger receptor in macrophages in vitro and in vivo. Immunology 103, 351–361 (2001).
Greaves, D.R., Quinn, C.M., Seldin, M.F. & Gordon, S. Functional comparison of the murine macrosialin and human CD68 promoters in macrophage and nonmacrophage cell lines. Genomics 54, 165–168 (1998).
Mariathasan, S. et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440, 228–232 (2006).
Leon, R.P. et al. Adenoviral-mediated gene transfer in lymphocytes. Proc. Natl. Acad. Sci. USA 95, 13159–13164 (1998).
Schaefer, B.C., Schaefer, M.L., Kappler, J.W., Marrack, P. & Kedl, R.M. Observation of antigen-dependent CD8+ T-cell/ dendritic cell interactions in vivo. Cell. Immunol. 214, 110–122 (2001).
Guarda, G. et al. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity 34, 213–223 (2011).
Kaser, A. et al. Interferon-alpha induces interleukin-18 binding protein in chronic hepatitis C patients. Clin. Exp. Immunol. 129, 332–338 (2002).
Sciacca, F.L., Canal, N. & Grimaldi, L.M. Induction of IL-1 receptor antagonist by interferon β: implication for the treatment of multiple sclerosis. J. Neurovirol. 6 (suppl. 2), S33–S37 (2000).
Pien, G.C., Satoskar, A.R., Takeda, K., Akira, S. & Biron, C.A. Cutting edge: selective IL-18 requirements for induction of compartmental IFN-γ responses during viral infection. J. Immunol. 165, 4787–4791 (2000).
Smith, H.R.C. et al. Recognition of a virus-encoded ligand by a natural killer cell activation receptor. Proc. Natl. Acad. Sci. USA 99, 8826–8831 (2002).
Andrews, D.M., Scalzo, A.A., Yokoyama, W.M., Smyth, M.J. & Degli-Esposti, M.A. Functional interactions between dendritic cells and NK cells during viral infection. Nat. Immunol. 4, 175–181 (2003).
McDonald, B. et al. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science 330, 362–366 (2010).
Pisetsky, D.S. The role of innate immunity in the induction of autoimmunity. Autoimmun. Rev. 8, 69 (2008).
González-Navajas, J.M., Lee, J., David, M. & Raz, E. Immunomodulatory functions of type I interferons. Nat. Rev. Immunol. 12, 125–135 (2012).
Jin, J. et al. LRRFIP2 negatively regulates NLRP3 inflammasome activation in macrophages by promoting Flightless-I-mediated caspase-1 inhibition. Nat. Commun. 4, 2075 (2013).
Yang, P. et al. The cytosolic nucleic acid sensor LRRFIP1 mediates the production of type I interferon via a β-catenin-dependent pathway. Nat. Immunol. 11, 487–494 (2010).
Khare, S. et al. An NLRP7-containing inflammasome mediates recognition of microbial lipopeptides in human macrophages. Immunity 36, 464–476 (2012).
Shao, R. & Guo, X. Human microvascular endothelial cells immortalized with human telomerase catalytic protein: a model for the study of in vitro angiogenesis. Biochem. Biophys. Res. Commun. 321, 788–794 (2004).
Bryan, N.B. et al. Differential splicing of the apoptosis-associated speck like protein containing a caspase recruitment domain (ASC) regulates inflammasomes. J. Inflamm. (Lond.) 7, 23 (2010).
Bryan, N.B., Dorfleutner, A., Rojanasakul, Y. & Stehlik, C. Activation of inflammasomes requires intracellular redistribution of the apoptotic speck-like protein containing a caspase recruitment domain. J. Immunol. 182, 3173–3182 (2009).
Fernandes-Alnemri, T. et al. The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 14, 1590–1604 (2007).
Gross, S. et al. Bioluminescence imaging of myeloperoxidase activity in vivo. Nat. Med. 15, 455–461 (2009).
Acknowledgements
We thank B.C. Schaefer (Uniformed Services University of the Health Sciences) for the promoter of the gene encoding ubiquitin C; J. DeGregori (University of Colorado Health Sciences Center) for the plasmid pLXS-hCARΔcyt; D. Trono (École Polytechnique Fédérale de Lausanne) for plasmids pMD2.G and psPAX2; K.A. Fitzgerald (University of Massachusetts) for Aim2−/− mice; the Northwestern University Transgenic and Targeted Mutagenesis Laboratory for assistance in generating transgenic mice; and A.D. Radian for the isolation of human macrophages. Supported by the US National Institutes of Health (GM071723, HL097183, AI092490, AI082406, AI099009 and AR064349 to C.S.; AR057532 to A.D., AR050250, AR054796, AI092490 and HL108795 to H.P.; AR064313 to C.M.C.; and T32AR007611 to L.d.A.), the National Cancer Institute (CA060553), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (AR057216), the American Heart Association (12GRNT12080035 to C.S.), the Arthritis Foundation (AF161715 to S.K.), the American Heart Association (11POST585000 to L.d.A.) and the Solovy/Arthritis Research Society (H.P.).
Author information
Authors and Affiliations
Contributions
A.D. and C.S. designed the research; S.K., R.A.R., L.d.A., C.M.C., S.L.R., A.V.M., M.C.W. and A.G. did experiments; E.F., E.G., H.P., J.C.R. and D.R.G. provided reagents, expertise and advice; S.K., R.A.R., L.d.A., C.M.C., A.V.M., H.P., A.D. and C.S. analyzed results; S.K., A.D. and C.S. wrote the paper; and A.D. and C.S. conceived of the study, designed the experiments and provided overall direction.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 POP3 is a previously undescribed gene located between IFI16 and IFIX.
(a) cDNA showing the open reading frame of POP3 (Genbank accession number: KF562078) (b) A nucleotide BLAST (blastn) analysis against the assembled human RefSeq genomes (http://blast.ncbi.nlm.nih.gov) detailing the genomic location of POP3 within the HIN-200 cluster flanked by IFI16 and PYHIN1 on human chromosome 1q23.
Supplementary Figure 2 POP3 shows characteristic features of PYDs present in HIN-200 proteins.
(a) Amino acid sequence of POP3. The PYD is shaded grey. The predicted α-helices are marked with blue lines (bottom), while the corresponding α-helices of AIM2, as determined by crystal structure1, are marked with a red line (top). (b) ClustalW alignment of the amino acid sequences corresponding to the PYDs of POP3 and human HIN-200 members. (c) ClustalW alignment of all human PYDs. The characteristic amino acid motifs found in HIN-200 members, which are also present in POP3, are highlighted in yellow. (d) Phylogenetic tree cluster analysis of sequences used in b. The HIN-200 cluster, which includes POP3 is highlighted in yellow.
Supplementary Figure 3 Silencing of POP3 specifically affects the AIM2 inflammasome.
(a, c, d) hMΦ were transfected with either control or a, POP3#2 or c, d, POP3 siRNAs and infected with MVA or transfected with poly(dA:dT) as indicated for 16 h and analyzed for a, mature IL-1β and c, IL-6 by ELISA (n = 3 ± s.e.m.) and d, TCL from Figure 3f, were analyzed in parallel for expression of AIM2 and IFI16 by immunoblot. (b, f) THP-1 cells were transfected with siRNAs as above, and infected with MVA, transfected with poly(dA:dT) or treated with MSU or SiO2, as indicated for 16 h and analyzed for b, IL-1β secretion and f, IFN-b by ELISA (n = 3 ± s.e.m.). (e, g) THP-1 (GFP) and THP-1 (GFP-POP3) cells were analyzed for secretion of e, TNFα and g, IFN-β in response to MVA and MCMV infection, transfection of poly(dA:dT) and treatment with MSU as indicated by ELISA (n = 3 ± s.e.m.). (h) The POP3 antibody does not cross-react with other POP family members. HEK293 cells were transfected with Myc-tagged POP1, POP2 and POP3 and immunoprobed with our custom POP1, POP2 and POP3-specific antibodies. (i) The POP3 antibody does not cross-react with the related PYDs of AIM2 and IFI16. HEK293 cells were transfected with GFP or RFP-tagged POP3, AIM2-PYD and IFI16-PYD and immunoprobed with our POP3 antibody and with GFP and RFP antibodies as control. * denotes a cross-reactive protein. Data are representative of 3 experiments (a), 2 experiments (b-d, f) and 1 experiment (e, g-i). (a) *P<0.0001, **P=0.0049; (b) *P=0.0199, **P=0.0495; (f) *P<0.0001; (g) *P=0.0031, **P=0.0161, ***P=0.0013;.
Supplementary Figure 4 Gating strategy for immunophenotyping of peripheral blood and peritoneal lavage cells.
(a) Peripheral blood cells and (b) peritoneal lavage cells obtained 6 h after MCMV infection were gated according to established cell surface markers, as indicated.
Supplementary Figure 5 Validation of POP3 function in mouse macrophages.
(a) Thioglycollate-elicited PM were isolated by peritoneal lavage, transfected with poly(dA:dT) for 16 h and analyzed for mature IL-1β by ELISA (n = 3 ± s.e.m.). (b) BMDM isolated from a 2nd line of CD68-POP3 TG mice were infected with MVA, treated with LPS/ATP or transfected with poly(dA:dT) for 16 h and analyzed for mature IL-1β by ELISA (n = 3 ± s.e.m.). (c) BMDM of UbiC-hCAR TG mice were immunoprobed for expression of hCARΔcyt using HEK293 cells transiently transfected with hCARΔcyt as a control. (d) WT (top panel) and UbiC-hCAR TG (bottom panel) BMDM were infected with increasing MOI of a GFP-expressing AdV and analyzed by fluorescence and phase contrast microscopy. (e) UbiC-hCAR TG BMDM were infected with low MOI of AdV expressing GFP or GFP-POP3 and transfected 48 h later with poly(dA:dT) or infected with MVA for 16 h and analyzed for secreted IL-1β by ELISA. (f) WT and POP3 transgenic BMDM were infected with MVA and analysed for mRNA expression of IL1ra and Il18bp (n=3 ± s.e.m.). Data are representative of two (a-c, e) and one (d, f) experiments. (b) ***P=0.0097, **P=0.0038; (e) ***P=0.0001, **P=0.0058; (f) *P=0.0029 (Il1ra); *P=0.0009 (Il18bp).
Supplementary Figure 6 Gating strategy for immunophenotyping of splenocytes.
Splenocytes obtained 36 h after MCMV infection were gated according to established cell surface markers, as indicated.
Supplementary Figure 7 POP3 does not ameliorate MSU-induced peritonitis.
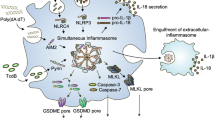
(a) WT and CD68-POP3 TG mice were i.p. injected with PBS or MSU crystals (10 mg/mouse) and mice were imaged for MPO activity in vivo 5 h later (n=3-7), showing representative examples. (b) Model of the type I IFN-induced regulatory loop of cytosolic DNA-induced inflammasome response that involves POP3. (a) Data are representative of one experiment.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 (PDF 3224 kb)
Rights and permissions
About this article
Cite this article
Khare, S., Ratsimandresy, R., de Almeida, L. et al. The PYRIN domain–only protein POP3 inhibits ALR inflammasomes and regulates responses to infection with DNA viruses. Nat Immunol 15, 343–353 (2014). https://doi.org/10.1038/ni.2829
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/ni.2829
This article is cited by
-
Type I interferon (IFN)-inducible Absent in Melanoma 2 proteins in neuroinflammation: implications for Alzheimer’s disease
Journal of Neuroinflammation (2019)
-
The AIM2 inflammasome is a central regulator of intestinal homeostasis through the IL-18/IL-22/STAT3 pathway
Cellular & Molecular Immunology (2017)
-
The PYRIN domain-only protein POP2 inhibits inflammasome priming and activation
Nature Communications (2017)
-
Pyrin-only protein 2 limits inflammation but improves protection against bacteria
Nature Communications (2017)
-
Assembly and regulation of ASC specks
Cellular and Molecular Life Sciences (2017)